Scheme 2.
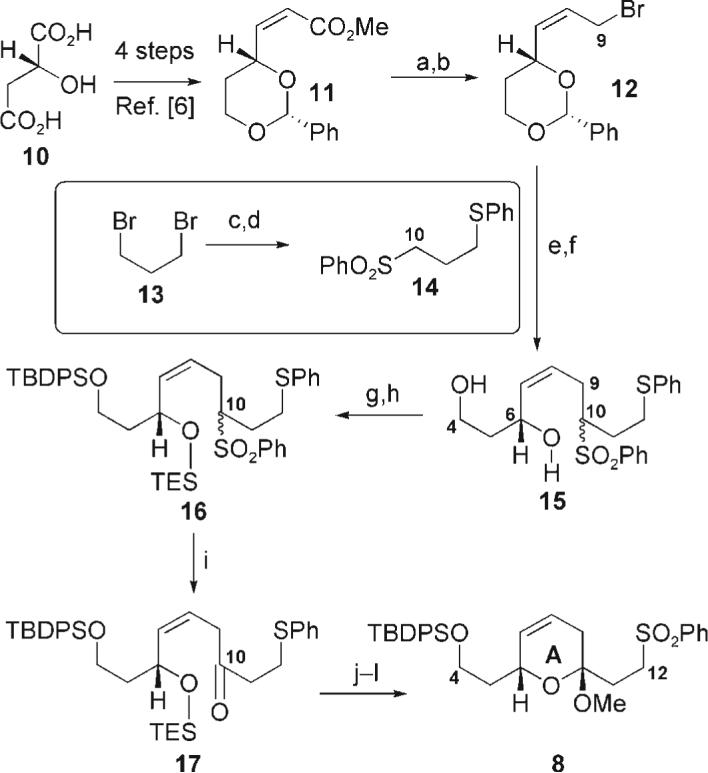
Synthesis of the sulfone. Reagents and conditions: a) DIBAL-H, CH2Cl2, 78°C, 98%; b) Ph3P, CBr4, MeCN, 92%; c) NaH, PhSH, DMF, 0°C, 73%; d) NaSO2Ph, DMF, 86%; e) nBuLi, 14 (2.6 equiv), THF, 78°C; f) 2 n HCl, MeCN, 58% (over two steps); g) TBDPSCl, imid., CH2Cl2, 92%; h) TESOTf, 2,6-lutidine, CH2Cl2, 95%; i) NaHMDS, THF then (TMSO)2, 72% (BORSM); j) NH4F, MeOH/CH2Cl2 (4:1); k) PPTS, MeOH, 68% (over two steps, BORSM); l) TPAP, NMO, MeCN, 40°C, 88%. DIBAL-H = diisobutylaluminum hydride; DMF = N,N-dimethylformamide; imid = imidazole; TESOTf = triethylsilyl triflate; HMDS = hexamethyldisilazide; TMS = trimethylsilyl; PPTS = pyridinium p-toluenesulfonate; TPAP = tetra-n-propylammonium perruthenate; NMO = N-methylmorpholine-N-oxide. BORSM = based on recovered starting material.
