FIGURE 2.
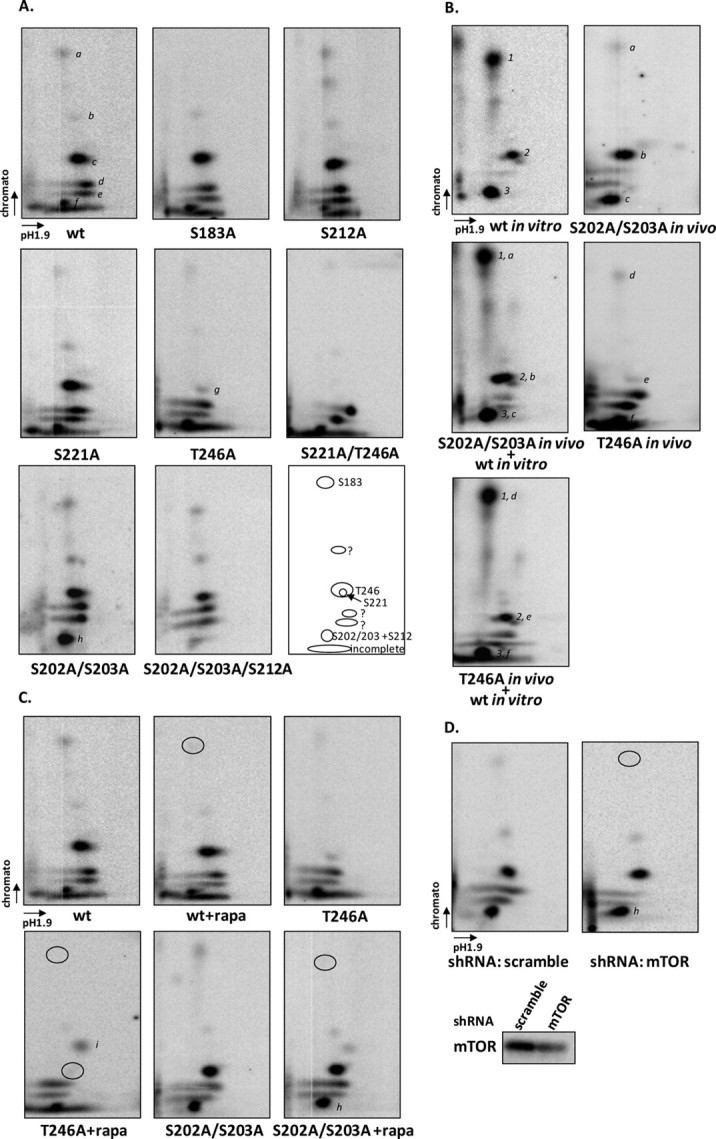
Phosphorylation of PRAS40 by mTORC1 in vivo. PRAS40 and mutants were transfected in HEK293 cells (A and B), and cells were labeled with [32P]orthophosphate for 3 h followed by insulin treatment (200 nm) for 30 min. 32P-Labeled PRAS40 was immunoprecipitated by HA antibody, and trypsin-digested peptides were resolved by two-dimensional phosphopeptide mapping. A, a variety of PRAS40 mutants were used to assess phosphopeptides. Major peptide spots are indicated by a–g, and phosphorylation sites are indicated in the last panel. B, in vitro and in vivo phosphorylated PRAS40 were mixed after trypsin digestion. 1, 2, and 3 indicate phosphopeptides obtained from in vitro phosphorylation by mTORC1 as described in Fig. 1C. The phosphopeptides in vivo are indicated by a–c in S202A/S203A (SS202,3AA) mutants and d–f in the T246A mutant. C, HEK293 cells were treated without or with rapamycin (rapa; 20 nm) for 20 min before insulin treatment. The circles indicate the spots that were altered after rapamycin treatment. D, HEK293T cells with lentivirus expressing either scramble or mTOR short hairpin RNA (shRNA) were transfected with PRAS40 mutant S202A/S203A. The circles indicate the spots that were altered in mTOR knockdown cells. wt, wild type.
