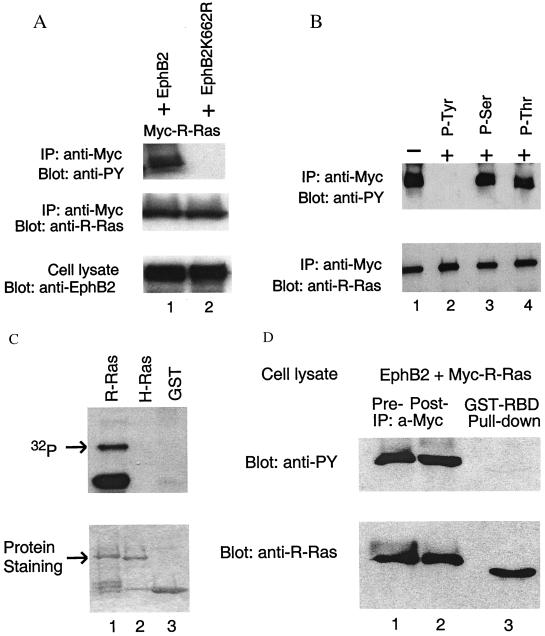
Figure 2.
R-Ras phosphorylation on tyrosine by EphB2 impairs binding to Raf-1. (A) Phosphorylation of R-Ras in cells expressing activated EphB2. Extracts of 293T cells cotransfected with EphB2, or EphB2K662R, and Myc-tagged R-Ras were subjected to immunoprecipitation (IP) with anti-Myc antibody. The immunoprecipitates, and cell lysates, were probed by immunoblotting as indicated. Anti-PY, anti-phosphotyrosine. (B) The labeling of R-Ras with anti-phosphotyrosine antibody is specific. R-Ras immunoprecipitated with anti-Myc antibody was probed with anti-phosphotyrosine antibody, or this antibody together with 1 mM phosphotyrosine, phosphoserine, or phosphothreonine. Only phosphotyrosine blocked antibody labeling of R-Ras. (C) Phosphorylation of R-Ras by EphB2 in vitro. Equal amounts of purified GST-R-Ras, GST-H-Ras, or GST alone were incubated with equal amounts of immunoprecipitated EphB2 in the presence of [γ-32P]ATP. Phosphorylation of R-Ras was analyzed by SDS/PAGE followed by autoradiography. The lower molecular weight phosphorylated band in the R-Ras lane is presumably a degradation product of GST-R-Ras. Coomassie blue protein staining shows the amount of each GST protein used in the assay. The arrows point to the Ras proteins. (D) Tyrosine-phosphorylated R-Ras lacks the ability to bind to Raf-1. Extracts of cells transfected with Myc-tagged R-Ras and EphB2 were incubated with the immobilized GST-Ras binding domain of Raf-1 (GST-RBD). Cell extracts and the supernatant of the GST-RBD incubation were immunoprecipitated with anti-Myc antibody. Immunoprecipitates (lanes 1 and 2) and proteins bound to GST-RBD (lane 3) were analyzed by immunoblotting as indicated. The large amount of GST-RBD in lane 3 interfered with the migration of the R-Ras band, making it appear of lower molecular weight than in the other lanes.