Abstract
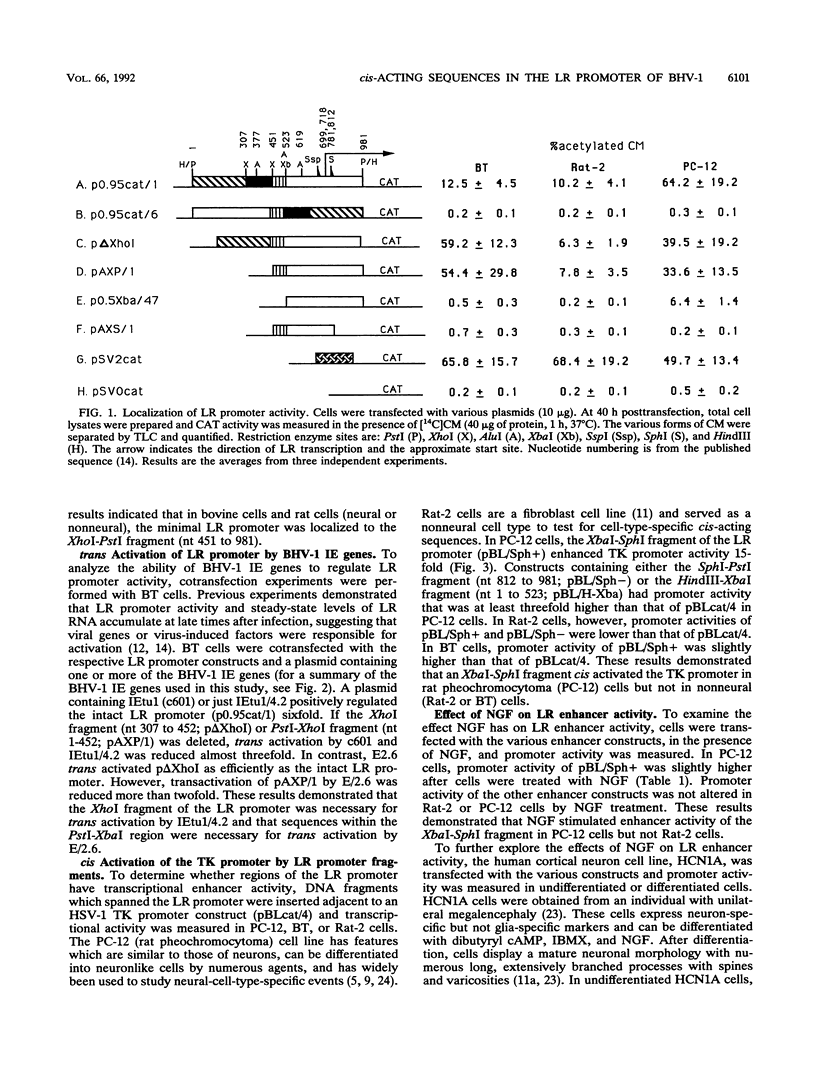
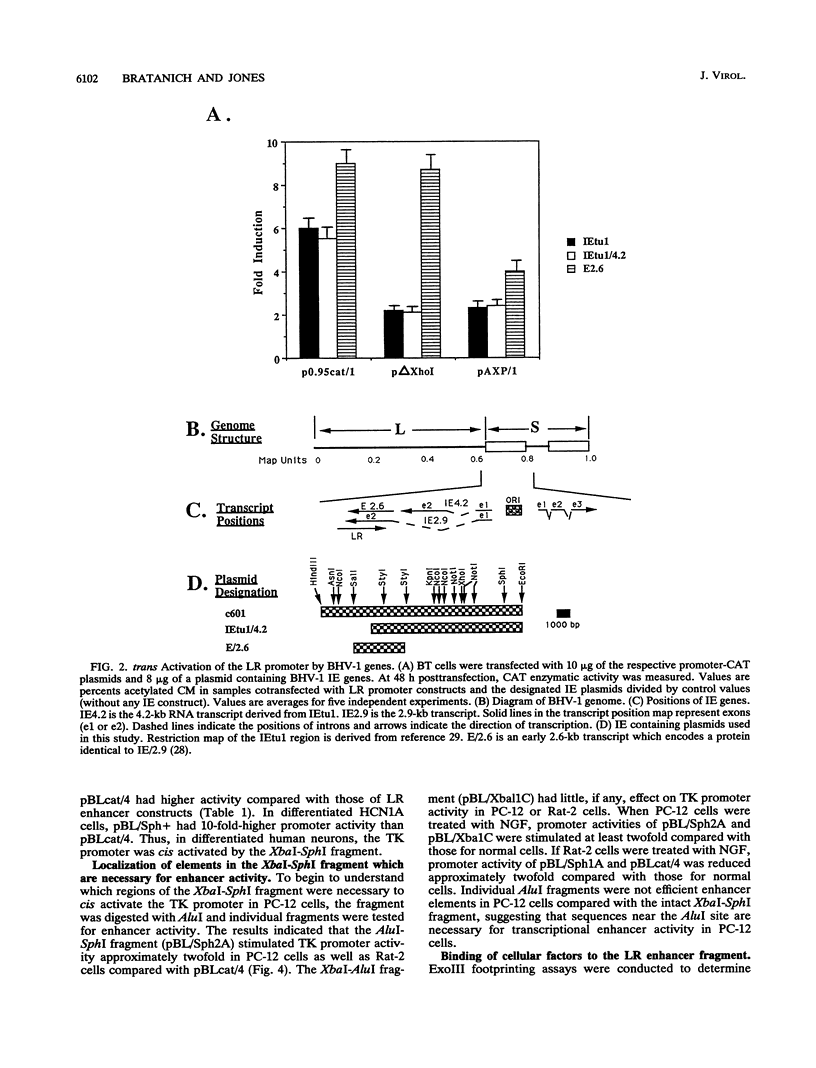
Bovine herpesvirus 1 (BHV-1) establishes a latent infection in sensory ganglionic neurons of cattle. During a latent infection, a single latency-related (LR) transcript is expressed. This observation suggested that DNA sequences in the LR promoter are positively regulated by neural cell type factors. The regulation of the LR gene was examined in neural cells as well as nonneural cells in transient assays. A 258-bp XbaI-SphI fragment from the LR promoter cis activated the herpes simplex virus type 1 thymidine kinase promoter in rat pheochromocytoma (PC-12) cells and differentiated human (HCN1A) neurons. In contrast, cis activation was not observed with rat (Rat-2) fibroblasts, undifferentiated HCN1A cells, or bovine turbinate cells. Treatment of PC-12 cells with nerve growth factor increased transcriptional activity of the XbaI-SphI fragment. Exonuclease III footprinting experiments suggested that nuclear factors bind to the XbaI-SphI fragment. The immediate-early genes of BHV-1 trans activated the LR promoter, and DNA sequences 5' to the XbaI-SphI fragment were necessary for maximal stimulation. These results imply that neural-cell-type-specific factors and BHV-1 immediate-early genes positively regulate LR gene expression.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann M., Peterhans E., Wyler R. DNA of bovine herpesvirus type 1 in the trigeminal ganglia of latently infected calves. Am J Vet Res. 1982 Jan;43(1):36–40. [PubMed] [Google Scholar]
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Batchelor A. H., O'Hare P. Localization of cis-acting sequence requirements in the promoter of the latency-associated transcript of herpes simplex virus type 1 required for cell-type-specific activity. J Virol. 1992 Jun;66(6):3573–3582. doi: 10.1128/jvi.66.6.3573-3582.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Crosby S. D., Puetz J. J., Simburger K. S., Fahrner T. J., Milbrandt J. The early response gene NGFI-C encodes a zinc finger transcriptional activator and is a member of the GCGGGGGCG (GSG) element-binding protein family. Mol Cell Biol. 1991 Aug;11(8):3835–3841. doi: 10.1128/mcb.11.8.3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent C. L., Lillycrop K. A., Estridge J. K., Thomas N. S., Latchman D. S. The B-cell and neuronal forms of the octamer-binding protein Oct-2 differ in DNA-binding specificity and functional activity. Mol Cell Biol. 1991 Aug;11(8):3925–3930. doi: 10.1128/mcb.11.8.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homan E. J., Easterday B. C. Experimental latent and recrudescent bovine herpesvirus-1 infections in calves. Am J Vet Res. 1983 Feb;44(2):309–313. [PubMed] [Google Scholar]
- Jones C., Delhon G., Bratanich A., Kutish G., Rock D. Analysis of the transcriptional promoter which regulates the latency-related transcript of bovine herpesvirus 1. J Virol. 1990 Mar;64(3):1164–1170. doi: 10.1128/jvi.64.3.1164-1170.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. The minimal transforming fragment of HSV-2 mtrIII can function as a complex promoter element. Virology. 1989 Apr;169(2):346–353. doi: 10.1016/0042-6822(89)90160-8. [DOI] [PubMed] [Google Scholar]
- Kemp L. M., Dent C. L., Latchman D. S. Octamer motif mediates transcriptional repression of HSV immediate-early genes and octamer-containing cellular promoters in neuronal cells. Neuron. 1990 Feb;4(2):215–222. doi: 10.1016/0896-6273(90)90096-x. [DOI] [PubMed] [Google Scholar]
- Kutish G., Mainprize T., Rock D. Characterization of the latency-related transcriptionally active region of the bovine herpesvirus 1 genome. J Virol. 1990 Dec;64(12):5730–5737. doi: 10.1128/jvi.64.12.5730-5737.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo D. C. NGF takes shape. Curr Biol. 1992 Feb;2(2):67–69. doi: 10.1016/0960-9822(92)90197-i. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Timmons P. M., Hébert J. M., Rigby P. W., Tjian R. Transcription factor AP-2 is expressed in neural crest cell lineages during mouse embryogenesis. Genes Dev. 1991 Jan;5(1):105–119. doi: 10.1101/gad.5.1.105. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Sevarino K. A., Wagner J. A., Mandel G., Goodman R. H. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhim J. S., Fujita J., Arnstein P., Aaronson S. A. Neoplastic conversion of human keratinocytes by adenovirus 12-SV40 virus and chemical carcinogens. Science. 1986 Apr 18;232(4748):385–388. doi: 10.1126/science.2421406. [DOI] [PubMed] [Google Scholar]
- Rock D. L., Beam S. L., Mayfield J. E. Mapping bovine herpesvirus type 1 latency-related RNA in trigeminal ganglia of latently infected rabbits. J Virol. 1987 Dec;61(12):3827–3831. doi: 10.1128/jvi.61.12.3827-3831.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock D. L., Hagemoser W. A., Osorio F. A., Reed D. E. Detection of bovine herpesvirus type 1 RNA in trigeminal ganglia of latently infected rabbits by in situ hybridization. J Gen Virol. 1986 Nov;67(Pt 11):2515–2520. doi: 10.1099/0022-1317-67-11-2515. [DOI] [PubMed] [Google Scholar]
- Rock D. L., Reed D. E. Persistent infection with bovine herpesvirus type 1: rabbit model. Infect Immun. 1982 Jan;35(1):371–373. doi: 10.1128/iai.35.1.371-373.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock D., Lokensgard J., Lewis T., Kutish G. Characterization of dexamethasone-induced reactivation of latent bovine herpesvirus 1. J Virol. 1992 Apr;66(4):2484–2490. doi: 10.1128/jvi.66.4.2484-2490.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnett G. V., Hester L. D., Nye J. S., Connors K., Snyder S. H. Human cortical neuronal cell line: establishment from a patient with unilateral megalencephaly. Science. 1990 May 4;248(4955):603–605. doi: 10.1126/science.1692158. [DOI] [PubMed] [Google Scholar]
- Salton S. R., Fischberg D. J., Dong K. W. Structure of the gene encoding VGF, a nervous system-specific mRNA that is rapidly and selectively induced by nerve growth factor in PC12 cells. Mol Cell Biol. 1991 May;11(5):2335–2349. doi: 10.1128/mcb.11.5.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein B., Rahmsdorf H. J., Steffen A., Litfin M., Herrlich P. UV-induced DNA damage is an intermediate step in UV-induced expression of human immunodeficiency virus type 1, collagenase, c-fos, and metallothionein. Mol Cell Biol. 1989 Nov;9(11):5169–5181. doi: 10.1128/mcb.9.11.5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T., Tjian R. Analysis of the DNA-binding and activation properties of the human transcription factor AP-2. Genes Dev. 1991 Apr;5(4):670–682. doi: 10.1101/gad.5.4.670. [DOI] [PubMed] [Google Scholar]
- Wirth U. V., Fraefel C., Vogt B., Vlcek C., Paces V., Schwyzer M. Immediate-early RNA 2.9 and early RNA 2.6 of bovine herpesvirus 1 are 3' coterminal and encode a putative zinc finger transactivator protein. J Virol. 1992 May;66(5):2763–2772. doi: 10.1128/jvi.66.5.2763-2772.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth U. V., Vogt B., Schwyzer M. The three major immediate-early transcripts of bovine herpesvirus 1 arise from two divergent and spliced transcription units. J Virol. 1991 Jan;65(1):195–205. doi: 10.1128/jvi.65.1.195-205.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. An exonuclease protection assay reveals heat-shock element and TATA box DNA-binding proteins in crude nuclear extracts. Nature. 1985 Sep 5;317(6032):84–87. doi: 10.1038/317084a0. [DOI] [PubMed] [Google Scholar]