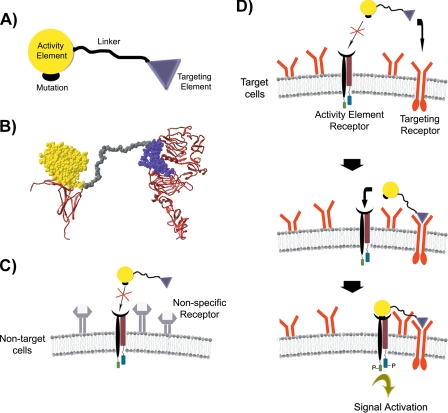
FIGURE 1.
A, general structure of chimeric activators, showing a targeting element connected by a peptide linker to an activity element with a mutation that reduces binding to the receptor for the activity element. B, molecular model of the IFNα-2a-EGF chimeric activator (space-filling structure), showing how the IFNα-2a and EGF components can simultaneously interact with their receptors (ribbons). Models for EGF·EGFR complex (12, 13), and the IFNα-2a·IFNAR2 complex (14, 15), are shown with the C termini of the receptor extracellular domains at the bottom; in each case, these C termini are followed by the membrane-spanning segment of the receptor. C and D, mechanism of specific binding of chimeric activators to target cells. C, the chimeric activator binds poorly to non-target cells because the intrinsic binding affinity of the mutant activity element to its receptor is low. D, in contrast, the targeting element binds to receptors on a target cell at a high rate. After the targeting element complexes with its receptor, the activity element is in a high local concentration relative to its receptor so that the activity element can then bind and stimulate signal transduction.