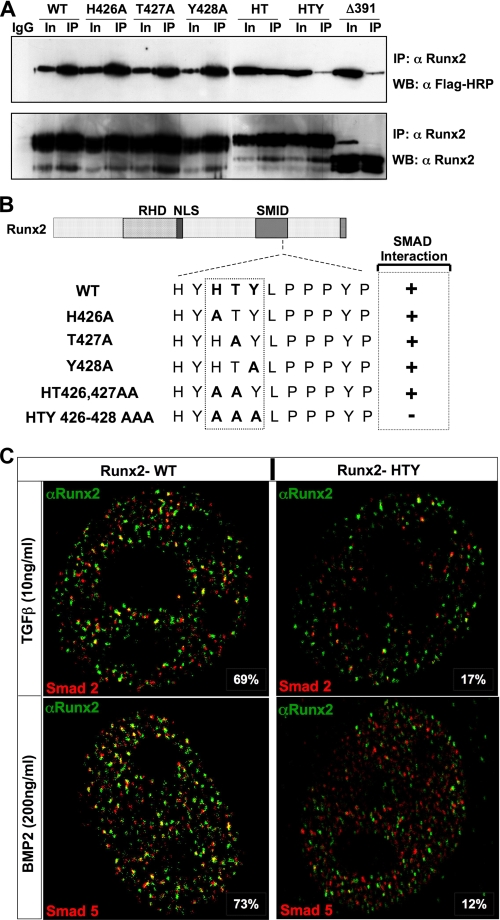
FIGURE 3.
Histidine, tyrosine, and threonine are the minimal contact points required for formation of the SMAD-Runx2 complex. A, HeLa cells were transiently co-transfected with 5 μg each of receptor Smad 1 and either wild-type or indicated Runx2 mutant plasmids using SuperFect transfection reagent. Cells were cultured in the presence of 100 ng/ml of BMP2 and harvested 24 h later. Immunoprecipitations were performed with polyclonal RUNX2 antibody as described under “Experimental Procedures.” HTY and Δ391 RUNX2 protein failed to co-immunoprecipitate SMAD1. B, summary of RUNX2-SMAD complex formation is indicated schematically. Loss of association is observed only for HTY mutant RUNX2 protein. C, HeLa cells were co-transfected with 1 μg of Smad2, Smad5, and wild-type or HTY mutant Runx2 expression plasmids. Cells were cultured in the presence of either 10 ng/ml TGFβ or 100 ng/ml BMP2 and processed 21 h later for in situ immunofluorescence. Ten cells from two independent coverslips with equal expression levels of both proteins were quantified for co-localizations of RUNX2 and SMAD foci by confocal microscopy, and a representative image is shown. The numbers represent the degree of co-localization observed for wild-type (69 and 73%) and HTY mutant (17 and 12%) proteins, respectively.