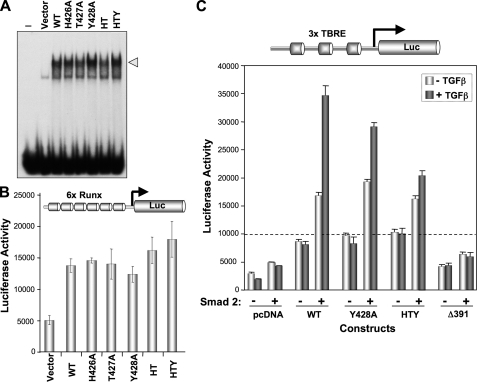
FIGURE 4.
Mutant RUNX2 proteins exhibit normal DNA binding and functional activation but not the integration of BMP/TGFβ signal. A, nuclear extracts (10 μg) prepared from transfected HeLa cells with indicated Runx2 plasmids were used for electrophoretic mobility shift assay. Comparable DNA binding activities indicated by the arrowhead are noted for all RUNX2 proteins. B, HeLa cells cultured in 6-well dishes were co-transfected with 0.4μg of pcDNA3.1 empty vector, wild-type, and mutant Runx2 plasmid and 1 μg of 6X-Runx-Luciferase reporter plasmids. Cells were harvested 24 h post-transfection, and luciferase activities were determined. Pooled data from two independent experiments (n = 12) are shown. C, cells were co-transfected with 0.5 μg of Smad2, 0.5 μg of indicated Runx2, 0.5 μg of 3X-TBRE-Luciferase reporter plasmids, and 100 ng of Renilla luciferase construct as an internal control. Each TGFβ response element in TBRE-luciferase reporter also contains an overlapping Runx binding element. Cells were treated with 5 ng of TGFβ for 6 h prior to harvest for luciferase assays. TGFβ stimulated enhanced (2- to 2.5-fold) promoter activation is lost by HTY and Δ391 RUNX2 mutant proteins.