Abstract
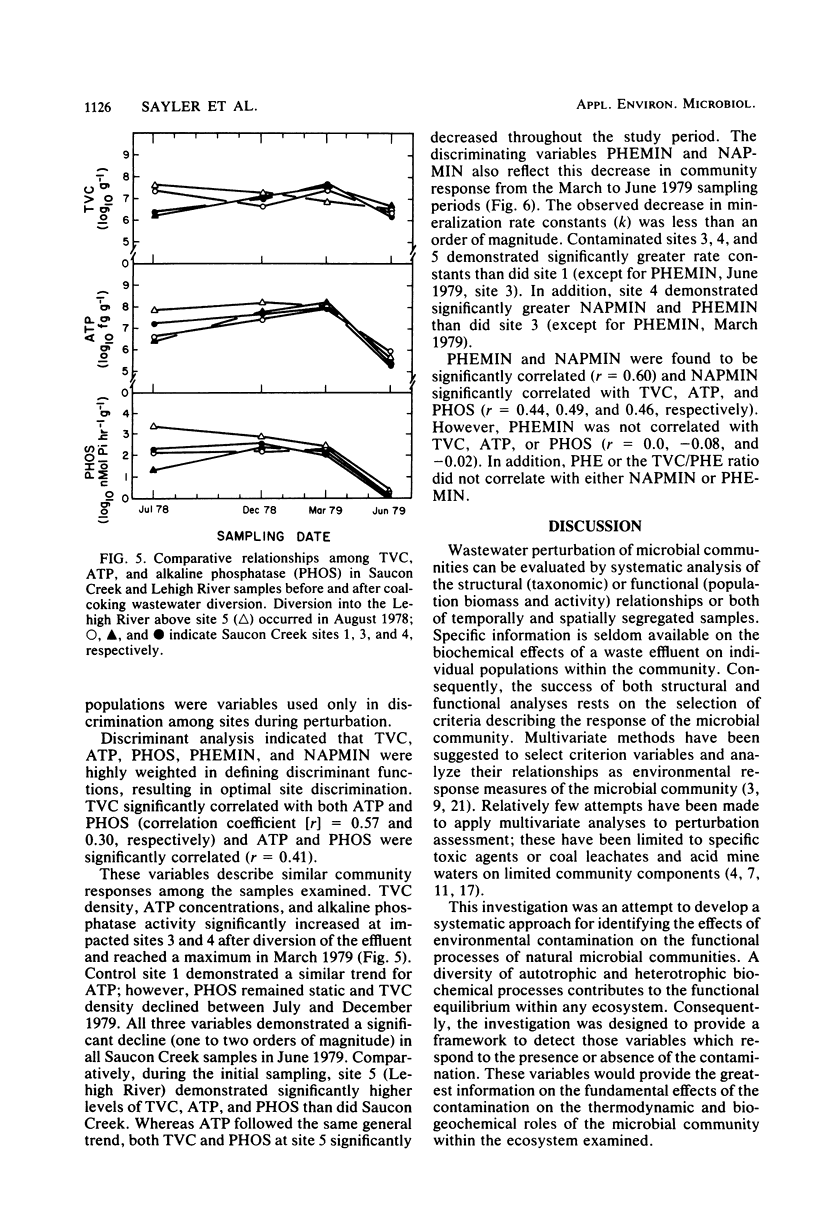
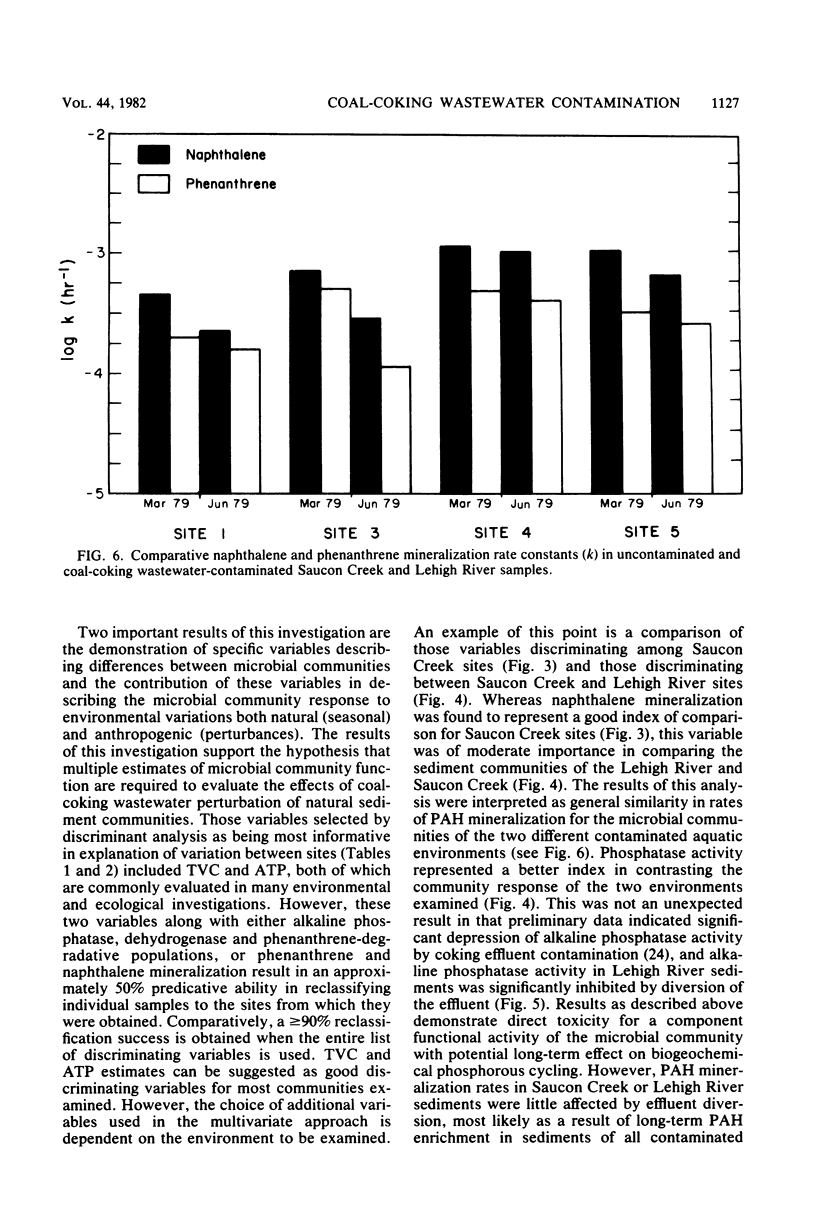
The functional response to and recovery from coal-coking waste effluent was evaluated for sediment microbial communities. Twenty estimates of microbial population density, biomass, and activity were measured five times during a 15-month period. Significant effects on microbial communities were observed in response to both wastewater contamination and diversion of the wastewater. Multivariate analysis of variance and discriminant analysis indicated that accurate differentiation between uncontaminated and contaminated sediments required a minimum of nine estimates of community response. Total viable population density, ATP, alkaline phosphatase, naphthalene, and phenanthrene mineralization rates were found to be highly weighted variables in site discrimination. Lipid and glucose mineralization, nitrogen fixation, and sediment protein also contributed significantly to explaining variation among sites. Estimates of anaerobic population densities and rates of methane production contributed little to discrimination among sites in the environment examined. In general, total viable population density, ATP, and alkaline phosphatase activity were significantly depressed in contaminated sediments. However, after removal of this contamination, the previously affected sites demonstrated greater temporal variability but a closer approximation of the mean response at the control site. Naphthalene and phenanthrene mineralization did not follow the general trend and were elevated at the contaminated sites throughout the investigation. Results of the investigation supported the hypothesis that multiple functional measures of microbial community response are required to evaluate the effect of and recovery from environmental contamination. In addition, when long-term effects are evaluated, select physiological traits, i.e., polyaromatic hydrocarbon mineralization, may not reflect population and biomass estimates of community response.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Herbes S. E. Rates of microbial transformation of polycyclic aromatic hydrocarbons in water and sediments in the vicinity of a coal-coking wastewater discharge. Appl Environ Microbiol. 1981 Jan;41(1):20–28. doi: 10.1128/aem.41.1.20-28.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. Appl Microbiol. 1974 May;27(5):985–987. doi: 10.1128/am.27.5.985-987.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen D., Sayler G. S. Methanogenesis in freshwater sediments: inherent variability and effects of environmental contaminants. Can J Microbiol. 1981 Feb;27(2):198–205. doi: 10.1139/m81-031. [DOI] [PubMed] [Google Scholar]
- Sayler G. S., Lund L. C., Shiaris M. P., Sherrill T. W., Perkins R. E. Comparative effects of Aroclor 1254 (polychlorinated biphenyls) and phenanthrene on glucose uptake by freshwater microbial populations. Appl Environ Microbiol. 1979 May;37(5):878–885. doi: 10.1128/aem.37.5.878-885.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayler G. S., Puziss M., Silver M. Alkaline phosphatase assay for freshwater sediments: application to perturbed sediment systems. Appl Environ Microbiol. 1979 Nov;38(5):922–927. doi: 10.1128/aem.38.5.922-927.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayler G. S., Shiaris M. P., Beck W., Held S. Effects of polychlorinated biphenyls and environmental biotransformation products on aquatic nitrification. Appl Environ Microbiol. 1982 Apr;43(4):949–952. doi: 10.1128/aem.43.4.949-952.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. D., Fitzgerald G. P., Burris R. H. In situ studies on N2 fixation using the acetylene reduction technique. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2071–2078. doi: 10.1073/pnas.58.5.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]


