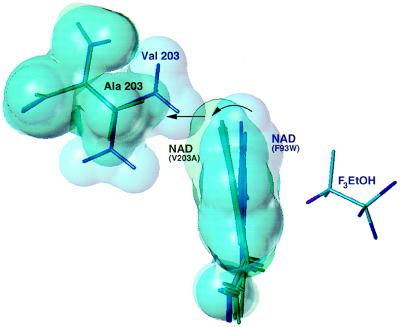
Figure 3.
Comparison of the four crystallographically independent nicotinamide rings in Val-203 → Ala (green) with the two independent rings in Phe-93 → Trp (blue). Surfaces are drawn at ≈90% of the van der Waals radii. Overlap of the structures is based on a least-squares alignment of the six independent cofactor binding domains. The nicotinamide rings are viewed edge-on along the glycosidic bond from the ribose, approximately normal to the view shown in Fig. 2. The nicotinamide ring in Phe-93 → Trp is in van der Waals contact with the “upper” methyl group of Val-203. In Val-203 → Ala, the nicotinamide ring rotates (curved arrow) to fill the gap left by replacement to alanine (straight arrow). Further rotation of the ring is prevented by steric contacts with Thr-178 (data not shown). Trifluoroethanol is displayed from the Phe-93 → Trp structure.