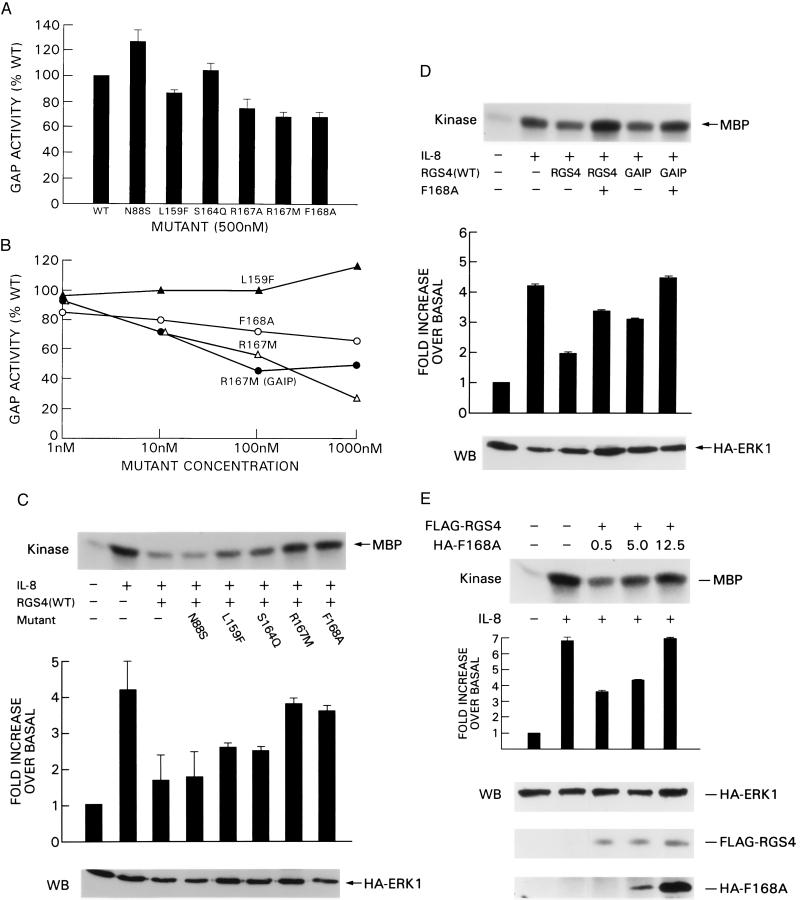
Figure 5.
Two mutants antagonize the activity of wild-type RGS4 and GAIP. (A) A 10-fold excess of mutant protein (500 nM) was added simultaneously with wild-type RGS4, and GTP hydrolysis by Giα1 was measured as previously. GTP hydrolyzed per minute by the G protein is represented in the bar graph as a percentage of the amount of GTP hydrolyzed in the presence of wild-type RGS4 alone (100%). Each experiment was repeated twice. (B) The GAP activity of wild-type RGS4 (or GAIP, as indicated) was determined in the presence of the indicated concentration of each mutant. Data are representative of two independent experiments. (C) F168A antagonizes the inhibition of IL-8-induced MAPK activation by RGS4 in vivo. A 5-fold excess of mutant plasmid over wild-type RGS4 was cotransfected into 6E cells, and MAPK activity was determined as previously described. Bar graph data represent the average value from 2–3 independent experiments. (D) F168A antagonizes the activity of both RGS4 and GAIP. In vitro kinase activity of HA immunoprecipitates from cells cotransfected with HA-ERK1, wild-type RGS4 or GAIP, and a 5-fold excess of F168A were determined as described in previous figures. Similar results were obtained with R167M or R167A. (E) Dose–response effect of increasing amounts of F168A. A constant amount of FLAG-RGS4 plasmid and increasing amounts of HA-F168A plasmid were coexpressed in 6E cells, and the in vitro kinase activity of MAPK after ligand stimulation was measured as described. Bar graph data represent two independent experiments. (Lower) Blots of cell lysates probed for ERK 1 (HA blot, Top), wild-type RGS4 (FLAG blot, Middle), and F168A (HA blot, Bottom).