Abstract
Nisin-controlled gene expression was used to develop a recombinant strain of Lactococcus lactis that is able to express the pneumococcal protective protein A (PppA) on its surface. Immunodetection assays confirmed that after the induction with nisin, the PppA antigen was predictably and efficiently displayed on the cell surface of the recombinant strain, which was termed L. lactis PppA. The production of mucosal and systemically specific antibodies in adult and young mice was evaluated after mice were nasally immunized with L. lactis PppA. Immunoglobulin M (IgM), IgG, and IgA anti-PppA antibodies were detected in the serum and bronchoalveolar lavage fluid of adult and young mice, which showed that PppA expressed in L. lactis was able to induce a strong mucosal and systemic immune response. Challenge survival experiments demonstrated that immunization with L. lactis PppA was able to increase resistance to systemic and respiratory infection with different pneumococcal serotypes, and passive immunization assays of naïve young mice demonstrated a direct correlation between anti-PppA antibodies and protection. The results presented in this study demonstrate three major characteristics of the effectiveness of nasal immunization with PppA expressed as a protein anchored to the cell wall of L. lactis: it elicited cross-protective immunity against different pneumococcal serotypes, it afforded protection against both systemic and respiratory challenges, and it induced protective immunity in mice of different ages.
Streptococcus pneumoniae is a common cause of invasive disease and respiratory tract infections in developed and developing countries. Risk groups for diseases caused by pneumococci include children in their first few years of life, elderly people, and patients with immunodeficiencies (7, 8). Because children under 2 years of age have an incompletely developed anatomy and an immature immune system, they are particularly vulnerable to pneumococcal infection by these bacteria (11). The rapid emergence of multidrug-resistant S. pneumoniae strains throughout the world has led to an increased emphasis on the prevention of pneumococcal infections by vaccination (19). However, available vaccines present disadvantages associated with their low immunogenicity in populations at risk (i.e., the pneumococcal 23-valent polysaccharide vaccine) or with their high cost as a public health strategy in developing countries (i.e., conjugated vaccine) (6, 7). Some pneumococcal surface proteins are serotype independent and represent a promising alternative for the design of a vaccine (9, 31). However, it seems probable that several pneumococcal proteins are necessary for the production of an effective vaccine against all serotypes. Thus, worldwide research on this subject is focused on the search for possible additional candidates that are antigenically conserved and that elicit antibodies that reduce colonization or protect against systemic disease or both. A novel pneumococcal surface protein, identified as pneumococcal protective protein A (PppA), has been described (15). This protein is antigenically conserved among different serotype strains of S. pneumoniae, and it has been reported that nasal immunization of adult mice with recombinant PppA administered with mucosal adjuvants elicits antibodies that are effective in reducing pneumococcal nasal colonization.
Lactic acid bacteria (LAB) are promising candidates as safe vehicles for the in vivo delivery of antigens. Compared with attenuated bacterial vectors, LAB are nonpathogenic and noninvasive gram-positive organisms that are generally recognized as safe. The best-known lactic acid bacterium, Lactococcus lactis, has been extensively engineered for the production of heterologous proteins (for a review see reference 20). Recombinant L. lactis expressing heterologous antigens has been used successfully to elicit an immune response against bacterial (10, 26) or viral antigens (12). In this sense, there are reports of pneumococcal antigens expressed by recombinant LAB which have been used to improve resistance against S. pneumoniae infection (2, 16, 29). In these experiments, nasal immunization was used to evaluate the efficacy of the experimental vaccines, since it has been demonstrated that nasal administration of antigens is an efficient route with which to elicit protective immunity in both the mucosal and the systemic immune compartments. However, immunization challenge experiments were performed with adult mice, despite the fact that young individuals are more susceptible to pneumococcal infection. Moreover, those authors used pneumococcal serotypes which are not the most common serotypes in our country (22), and the efficacy of the experimental vaccines against different pneumococcal serotypes was not evaluated. Thus, in the present work, we carried out experiments better suited to our local conditions: we expressed in L. lactis NZ9000 a recently characterized antigen, PppA, and assessed its efficacy to induce local and systemic immune responses in mice of different ages. In addition, we determined whether the mucosal administration of the recombinant bacteria increased resistance to systemic and mucosal infections caused by the main S. pneumoniae serotypes found in our country (22).
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Serotype 14 Streptococcus pneumoniae T14 (Table 1) was isolated from the blood of a patient at the Niño Jesús Children's Hospital in Tucumán, Argentina, and serotyped at the Administración Nacional de Laboratorios e Institutos de Salud, Buenos Aires, Argentina. Streptococcus pneumoniae T14 was grown in microanaerobiosis in Todd-Hewitt broth at 37°C. Escherichia coli DH10B and E. coli BL21(D3) (Novagen) were grown with shaking in LB medium at 37°C, and Lactococcus lactis NZ9000 was grown in M17 medium supplemented with 1% glucose (M17-glu) at 30°C. CaCl2-competent E. coli cells were transformed as described by Sambrook et al. (28), and transformants were selected in LB agar (1.2%) containing neomycin (50 μg/ml; Sigma). Electroporation of L. lactis was conducted as indicated by Le Loir et al. (20), and transformants were selected in M17-glu agar (1.2%) with erythromycin (5 μg/ml; Sigma).
TABLE 1.
Strains of Streptococcus pneumoniae used in this study
| S. pneumoniae strain | Capsule type | Source | Reference |
|---|---|---|---|
| T14 | 14 | Blood | 26 |
| AV3 | 3 | CSFa | This work |
| AV6 | 6B | Blood | This work |
| AV14 | 14 | Blood | This work |
| AV23 | 23F | CSF | This work |
CSF, cerebrospinal fluid.
Cloning and expression of recombinant PppA in E. coli and L. lactis.
All cloning techniques were carried out as described by Sambrook et al. (28). Restriction endonuclease and ligation reactions were carried out according to the recommendations of the suppliers. The pppA gene was amplified from the chromosomal DNA of S. pneumoniae T14 (Table 1) with the primers EC1 and EC2 (Table 2), following the conditions described by Green et al. (15). PCR amplifications were performed with a DNA thermocycler (MyCycler 580BR; Bio-Rad) by using 28 cycles consisting of a denaturation step at 94°C for 60 s, a primer annealing step at 52°C for 60 s, and a primer extension step at 72°C for 60 s. The PCR product was purified from agarose gels, using homemade silica beads, and treated with the restriction enzymes NcoI and SalI. Then, the pppA gene was ligated into the NcoI and SalI sites of pET28b, using T4 DNA ligase (Promega). A recombinant plasmid containing the pppA gene, named pET-PppA, was first recovered into E. coli DH10B cells and then transformed into BL21(DE3) cells for expression. BL21(DE3)(pET-PppA) cells were grown with aeration until they reached an optical density at 600 nm (OD600) of 0.8 in LB medium supplemented with neomycin (50 μg/ml) and induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) (Sigma) for 2 to 4 h. Whole-cell lysates were prepared as indicated by Hebert et al. (18). Cell debris was removed by centrifugation (20 min, 13,000 rpm, 4°C), and the proteins present in the supernatant were run on a 12% sodium dodecyl sulfate (SDS)-polyacrylamide gel to confirm expression of the recombinant PppA (rPppA). rPppA was then purified by using a His-Bind purification kit (Novagen) according to the recommendations of the supplier and visualized by electrophoresis on 12% SDS-polyacrylamide gels. For expression of the pppA gene in L. lactis, primers LL1 and LL2 were designed (Table 2) and used to amplify, under the same conditions as indicated above (15), pppA sequences from the chromosomal DNA of S. pneumoniae T14. The PCR product was then treated with the restriction enzymes SalI and EcoRV and subcloned into the SalI and EcoRV sites of pVE5547 (12). Electrocompetent L. lactis NZ9000 cells were transformed with the ligation mixture, and a recombinant strain containing plasmid pVE-PppA was selected and named L. lactis PppA. Recombinant L. lactis PppA was grown in M17-glu plus erythromycin (5 μg/ml) at 30°C until cells reached an OD590 of 0.6 and were then induced with 50 ng/ml of nisin for 2 h. Uninduced (L. lactis PppA) and induced (L. lactis PppA expressing the PppA protein on its cell surface [PppA+]) strains were then used in the immunization experiments. Sequencing of the reactions to confirm the identity of the plasmids pET-PppA and pVE-PppA was carried out at the Sequence Service Facility of GAD, National University of La Plata (Buenos Aires, Argentina).
TABLE 2.
Sequences of oligonucleotide primers used for PCR amplification and cloning of the pppA gene
| Primer | Sequence (5′-3′)a | Restriction site | Reference |
|---|---|---|---|
| EC1 (forward) | GGGGCCATGGCTTGTAGAATTGAAAAAAGAA | NcoI | 16 |
| EC2 (reverse) | GGGGTCGACTAAACCAGGTGCTTGTCCAAGTTC | SalI | 16 |
| LL1 (forward) | GGGGGTCGACGTAGAATTGAAAAAAGAA | SalI | This work |
| LL2 (reverse) | GGGGATATCTCTAAACCAGGTGCTTGTCCAAGTTCb | EcoRV | This work |
Restriction sites in each primer are in boldface.
The underlined bases T and C in primer LL2 were included to maintain an in-frame reading of the PppA and cell wall anchor proteins.
Western blotting analysis.
Purified rPppA protein was used to generate polyclonal antisera in mice. Briefly, 10 μg of rPppA protein was mixed with 50 μl of Freund's complete adjuvant and injected subcutaneously into 20 6-week-old Swiss albino mice (CERELA). The animals were immunized at week 0, given a booster injection at week 4, and then exsanguinated at week 6. The serum samples were pooled and used for further Western blotting analyses.
Whole-cell L. lactis PppA lysates were prepared as follows. Cells (5 × 108) were pelleted by centrifugation at 5,000 × g for 15 min, and then the supernatant and the pellet were collected. The pellet was resuspended in 500 μl of 50 mM Tris-HCl (pH 7.4), and cell extracts were prepared by adding glass beads (0.15- to 0.25-mm diameter; Sigma) to the bacterial cell suspensions and mixing the suspensions for 7 min at 4°C in a vortex mixer (Reax 2000; Heidolph, Schwabach, Germany) at maximum speed. Glass beads, cell debris, and unbroken cells were removed by centrifugation (10,000 × g, 10 min, 4°C), and the lysates were recovered. For protein secretion analysis, 1 ml of supernatant was concentrated by precipitation by the addition of 100 μl of 100% trichloroacetic acid, incubated for 10 min on ice, and centrifuged for 10 min at 7,000 × g.
Proteins in lysates and supernatants were separated by a 12% SDS-polyacrylamide gel and then transferred to nitrocellulose (Bio-Rad), using a Mini-Protean 3 cell unit (Bio-Rad). The blots were blocked at room temperature for 30 min in 5% nonfat milk-phosphate-buffered saline (PBS). Membranes were then exposed to pooled mouse antisera (at a 1:500 dilution) for 60 min, followed by three 20-min washes in PBS-0.2% Tween 20 (PBS-T). Peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) (anti-γ chain specific; Sigma-Aldrich) was diluted 1:500 and used to detect bound antibodies. The blots were washed, and peroxidase activity was detected with a 3,3′-diaminobenzidine peroxidase substrate solution (0.05% 3,3′-diaminobenzidine tetrahydrochloride, 0.015% H2O2 in 0.01 M PBS [pH 7.2]; Sigma).
Immunofluorescence microscopy.
Recombinant L. lactis PppA and PppA+ cells were grown as described previously. For immunofluorescence microscopy, the cell pellets were labeled sequentially with mouse anti-PppA (1:500) polyclonal antibodies and fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG (1:1,000) antibodies and then examined by using a fluorescence microscope (Leica DM LS2).
Immunization of mice.
Three-week-old (young) and 6-week-old (adult) Swiss albino mice (CERELA) were nasally immunized with 108 cells/mouse of recombinant L. lactis PppA+ at days 0, 14, and 28 (10 animals per group). The inoculum was slowly instilled into the nostril of each mouse in a 25-μl volume. Negative controls included uninduced L. lactis PppA and PBS. Serum and bronchoalveolar lavage (BAL) fluid samples were collected on days 0, 14, 28, and 42 for the determination of specific antibodies. BAL samples were obtained according to the technique described previously (33); briefly, the trachea was exposed and intubated with a catheter, and two sequential bronchoalveolar lavage steps were performed by injecting 0.5 ml of sterile PBS into each mouse. The sample of fluid was centrifuged for 10 min at 900 × g, and the supernatant fluid was frozen at −70°C for subsequent antibody analyses.
Systemic challenge of actively and passively immunized mice.
Intraperitoneal challenge experiments were carried out 2 weeks after the third immunization of the animals. S. pneumoniae T14 was grown until cells reached their exponential phase under the conditions described above, and each mouse was infected with 100 μl of PBS containing 108 cells of the pathogen. Survival was closely monitored for 21 days. In addition, two types of passive immunization and challenge experiments were performed as described by Gor et al. (14). In the first series of experiments, the groups of mice to be challenged were passively immunized with an intraperitoneal injection of 100 μl of the pooled serum from adult or young mice previously immunized with L. lactis PppA+, L. lactis PppA, or PBS. Twenty-four hours after mice were passively immunized, they were challenged intraperitoneally with 108 cells of S. pneumoniae T14 suspended in PBS, and survival was monitored for 21 days. In a second series of experiments, groups of mice were inoculated with 108 cells of S. pneumoniae T14 suspended in 100 μl of PBS containing 10% of pooled serum from adult or young mice previously immunized with L. lactis PppA+, L. lactis PppA, or PBS, and survival was monitored for 21 days.
Respiratory challenge of actively immunized mice.
Adult and young mice were challenged with different serotypes of S. pneumoniae, which were kindly provided by M. Regueira from the Laboratory of Clinical Bacteriology, National Institute of Infectious Diseases, Argentina. Freshly grown colonies of S. pneumoniae strains AV3, AV6, AV14, and AV23 (Table 1) were suspended in Todd-Hewitt broth and incubated at 37°C until the log phase was reached. The pathogens were harvested by centrifugation at 3,600 × g for 10 min at 4°C and washed three times with sterile PBS; cell density was adjusted to 4 × 107 cells/ml. Challenge with the different pneumococcal strains was performed on day 42 postimmunization. Mice were challenged nasally with the pathogen by dripping 25 μl of an inoculum containing 106 cells into each nostril. To facilitate migration of the inoculum to the alveoli, mice were held in a head-up vertical position for 2 min. The development of pneumococcal disease was evaluated as previously described (25, 33). Briefly, mice were sacrificed 48 h after receiving the challenge, and their lungs were excised, weighed, and homogenized in 5 ml of sterile peptone water. Homogenates were diluted appropriately, plated in duplicate on blood agar, and incubated for 18 h at 37°C. S. pneumoniae colonies were counted, and the results were expressed as log10 CFU/g of organ. The progression of bacterial growth to the bloodstream was monitored by obtaining blood samples by using cardiac puncture with a heparinized syringe. Samples were plated on blood agar, and bacteremia was reported as negative or positive hemocultures after incubation for 18 h at 37°C.
ELISA for PppA antibodies.
Serum and BAL fluid antibodies against PppA protein were determined by a modification of the enzyme-linked immunosorbent assay (ELISA) protocol described by Green et al. (15). Briefly, plates were coated with rPppA (100 μl of a 5-μg/ml stock in sodium carbonate-bicarbonate buffer [pH 9.6] per well). Nonspecific protein binding sites were blocked with PBS containing 5% nonfat milk. Samples were diluted (serum, 1:100; BAL, 1:20) with PBS containing 0.05% (vol/vol) Tween 20 (PBS-T). Peroxidase-conjugated goat anti-mouse IgM, IgA, IgG, IgG1, or IgG2a (Fc specific; Sigma Chemical) was diluted (1:500) in PBS-T. Antibodies were revealed with a substrate solution (3-3′,5-5′-tetramethylbenzidine [Sigma Chemical]) in citrate-phosphate buffer (pH 5; containing 0.05% H2O2), and the reaction was stopped by the addition of H2SO4 1 M. OD readings were carried out at 493 nm (VERSAmax Tunable microplate reader), and samples were considered negative for the presence of specific antibodies when OD493 was <0.1.
Antibody avidity assay.
For the measurement of IgG and IgA antibody avidity, the basic ELISA method was used. After samples were incubated, plates were washed and incubated for 15 min at room temperature with 0.5 M sodium thiocyanate (NaSCN) to induce the dissociation of the antigen-antibody complexes. Plates were washed, and the remaining incubations were performed as described above, without modifications. The avidity index for each sample was determined as follows: the antibody concentration in the presence of the chaotropic agent NaSCN was divided by the antibody concentration in the absence of NaSCN and multiplied by 100 (1).
Statistical analysis.
Experiments were performed in triplicate, and results were expressed as means ± standard deviations. For body weight gain, one-way analysis of variance was used. For all other determinations, two-way analysis of variance was used. Tukey's test (for pairwise comparisons of the mean values of the different groups) was used to test for differences between the groups. Differences were considered significant at a P value of <0.05. For survival analysis, the Kaplan-Meier log-rank test was used. Differences were considered significant at a P value of <0.01. In order to compare survival rates between young and adult mice, Cox's F test and Gehay's Wilcoxon test were used.
RESULTS
Cloning and expression of PppA in E. coli.
A 534-bp DNA fragment containing the pppA gene of S. pneumoniae T14 was amplified with the primers EC1 and EC2 (Table 2), as described by Green et al. (15). The purified PCR product was cloned into the NcoI and SalI sites of plasmid pET28b to generate pET-PppA, which was recovered by transformation into E. coli DH10B. The identity of the pppA region present in pET-PppA was confirmed by sequencing. pET-PppA was then transferred to E. coli BL21(D3), and the recombinant strain E. coli BL21(D3)(pET-PppA) was used to express the rPppA (Fig. 1A). rPppA, a protein of 188 amino acids, with a histidine tag in its carboxyl ends and with a molecular mass of approximately 21 kDa, visualized only when recombinant E. coli BL21(D3)(pET-PppA) was induced with 1 mM IPTG (Fig. 2A). Purified rPppA (Fig. 2B) was used to obtain polyclonal anti-PppA serum in mice, which was then used for Western blotting and immunofluorescence analyses.
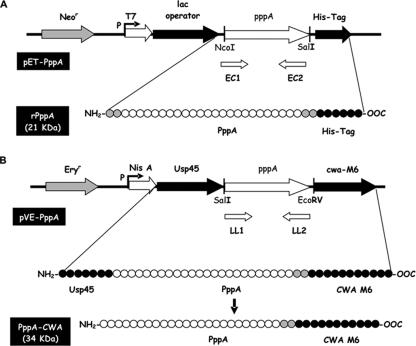
FIG. 1.
Plasmids constructed in this work and the expected products. (A) Plasmid pET-PppA derived from the insertion of the pppA gene in the NcoI-SalI site of the pET28b vector, which allows the expression of the rPppA in E. coli. rPppA has a histidine tag (His-Tag) that allows its purification. The expected molecular mass of rPppA is 21 kDa. (B) Plasmid pVE-PppA derived from the insertion of the pppA gene in the SalI-EcoRV site of the pVE5547 vector, which allows expression of the recombinant protein PppA-CWA in L. lactis. The recombinant protein is synthesized as a primary transcript with the secretion signal Usp45, which is processed to originate the PppA-CWA protein that is anchored to the cell wall by the anchoring signal CWA-M6. The expected molecular mass of PppA-CWA is 34 kDa.
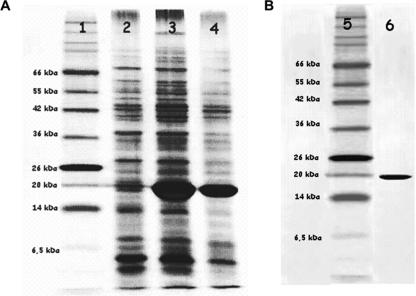
FIG. 2.
SDS-polyacrylamide gel electrophoresis (PAGE) analysis of (A) recombinant E. coli(pET-PppA) whole-cell lysates before and after induction with IPTG and (B) purified rPppA protein. Samples were loaded on a 12% SDS-polyacrylamide gel and the gel was silver stained. Lanes 1 and 5, molecular mass standards; 2, noninduced E. coli; 3, induction for 4 h; 4, induction for 2 h; 6, purified rPppA.
Expression of PppA in L. lactis NZ9000.
To facilitate cloning of the pppA gene into the pVE5547 expression vector, two new primers, LL1 and LL2, were designed (Table 2). The primer LL1 incorporates a SalI restriction site at its 5′ end, while the primer LL2 incorporates an EcoRV restriction site and two extra bases (T and C [Table 2, underlined in the sequence]) at its 5′ end to ensure that the pppA DNA sequence was in-frame with the cell wall spanning the domain sequence present in pVE5547. The 534-bp pppA DNA fragment was obtained by PCR amplification using chromosomal DNA from the S. pneumoniae T14 strain as a template. The purified PCR fragment was treated with restriction enzymes and cloned into the SalI and EcoRV sites of pVE5547 to generate pVE-PppA (Fig. 1B), which was recovered into the L. lactis expression host strain NZ9000 by electroporation. The identity of the recombinant plasmid pVE-PppA was confirmed by PCR analysis (Fig. 3A) and DNA sequencing. When the expression strain L. lactis PppA (harboring pVE-PppA) was grown and induced as indicated in Materials and Methods, a fusion protein of the correct size (34 kDa; 314 amino acids) was recognized by anti-PppA antibodies. Immunoblotting experiments showed that, as expected, the PppA-CWA fusion protein was detected only in the cell wall fraction of the induced L. lactis PppA (Fig. 3B). The PppA band was not detectable in the soluble cytoplasmic or extracellular fractions or in the respective uninduced recombinant strain (Fig. 3B). The localization of PppA on the surface of L. lactis PppA was further verified by immunofluorescence staining that revealed fluorescence only in the previously induced recombinant bacteria harboring pVE-PppA (Fig. 3C) but not in control cells (photos not shown). These results showed that PppA protein was predictably and efficiently displayed on the cell surface of L. lactis after it was induced with nisin.
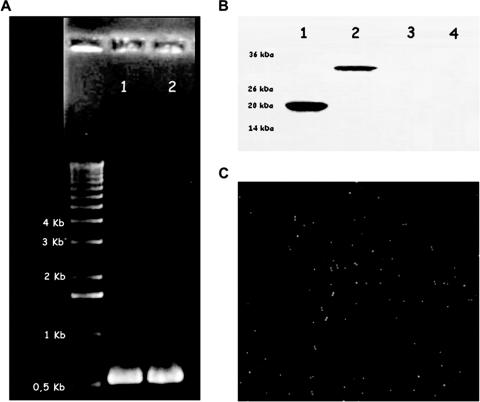
FIG. 3.
(A) A fragment of the 534 bp of the pppA gene amplified with the boosters LL1 and LL2 from the chromosomal DNA of Streptococcus pneumoniae T14 (lane 1) and of the pEV-PppA plasmid obtained from the erythromycin-resistant recombinant strain L. lactis PppA (lane 2). (B) Western blotting analysis of whole-cell lysates and culture supernatants of L. lactis PppA. An SDS-polyacrylamide gel was blotted onto nitrocellulose for analysis. Polyclonal mouse antiserum to rPppA was used as the primary antibody. Lanes 1, purified rPppA (21 kDa) from E. coli; 2, L. lactis PppA previously induced with nisin expressing the PppA-CWA protein (34 kDa); 3, L. lactis PppA without induction; 4, concentrated culture supernatants from induced L. lactis PppA. (C) Detection of PppA at the cell surface of L. lactis PppA by immunofluorescence. Recombinant L. lactis cells expressing the PppA-CWA protein were treated with specific polyclonal mouse antiserum and then fluorescently stained with goat anti-IgG conjugated with FITC. Magnification, ×100.
Nasal immunization with L. lactis PppA+ induced local and systemic antibody responses.
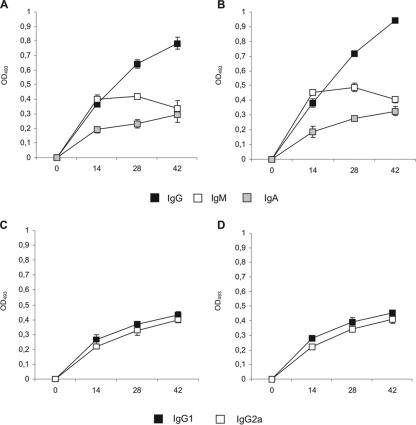
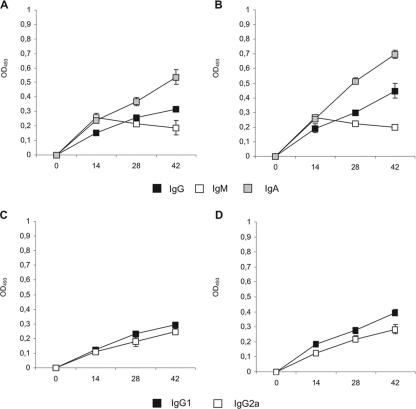
We evaluated whether nasal immunization with recombinant L. lactis PppA+ was able to induce the production of specific antibodies in adult and young mice. For this purpose, we used the immunization protocol described by Green et al. (15), which was shown to elicit strong antigen-specific IgG and IgA antibody responses in bronchial wash fluid and sera. Each mouse received one dose of 108 induced recombinant L. lactis PppA+, followed by two booster injections on days 14 and 28. Uninduced recombinant L. lactis PppA or PBS was used as control. No preimmune reactivity (day 0) was detected with PppA in either adult or young mice (OD493, <0.1). Samples analyzed on days 14, 28, and 42 showed that nasal immunization of young and adult mice with L. lactis PppA+ induced specific anti-PppA IgM, IgA, and IgG antibodies in both sera and BAL fluids (Fig. 4A and B and Fig. 5A and B), while no detectable values of these specific antibodies were observed with sera or BAL fluids collected from animals that received L. lactis PppA or PBS (data not shown). Anti-PppA serum IgG (P = 0.003) and BAL fluid IgA (P = 0.005) and IgG (P = 0.015) antibodies were significantly higher in immunized young mice than in the adult group (Fig. 4A and B and Fig. 5A and B). The avidity of anti-PppA IgM, IgG, and IgA antibodies was also determined in sera and BAL samples (Table 3), with no significant differences observed between the avidity of young mice and that of adult mice. In addition, the subclasses of IgG anti-PppA antibodies were studied. The production of both IgG1 and IgG2a anti-PppA antibodies was detected in the sera and BAL fluid of young and adult mice (Fig. 4C and D and Fig. 5C and D). To assess the safety of L. lactis PppA administration in adult and young mice, weight gain and the number of deaths were monitored daily and compared to those of age-matched mice in the PBS control groups. No deaths occurred, and no effect was observed with weight gain in adult or young mice immunized with L. lactis PppA+ or PppA compared to that of PBS controls (data not shown), indicating that the recombinant strain is safe even for subjects in early life.
FIG. 4.
Antibody immune response to PppA antigen in serum after nasal immunization of adult (A and C) or young (B and D) mice with recombinant L. lactis PppA+ (previously induced with nisin). Samples were considered negative for the presence of specific antibodies when OD493 ≤ 0.1.
FIG. 5.
Antibody immune response to PppA antigen in BAL fluid samples from nasally immunized adult (A and C) or young (B and D) mice with recombinant L. lactis PppA+ (previously induced with nisin). Samples were considered negative for the presence of specific antibodies when the OD493 was ≤0.1.
TABLE 3.
Avidity of PppA-specific antibodies in sera and BAL fluid samples after nasal immunization of adult and young micea
| Group | Avidity index ± SD response to serum antibodies
|
Avidity index ± SD response to BAL fluid antibodies
|
||||
|---|---|---|---|---|---|---|
| IgG | IgM | IgA | IgG | IgM | IgA | |
| Adult mice | ||||||
| L. lactis PppA+ | 62.3 ± 2.8 | 44.5 ± 1.9 | 18.3 ± 1.7 | 61.8 ± 3.9 | 56.7 ± 3.6 | 68.4 ± 4.7 |
| Young mice | ||||||
| L. lactis PppA+ | 64.1 ± 6.8 | 45.4 ± 2.3 | 21.5 ± 2.1 | 68.5 ± 3.4 | 57.8 ± 5.3 | 66.9 ± 3.8 |
Avidity of PppA-specific antibodies in sera and BAL fluid samples after nasal immunization of adult or young mice with recombinant L. lactis PppA+ previous to induction with nisin. The avidity index for each sample was determined by dividing the antibody concentration in the presence of the chaotropic agent NaSCN by the antibody concentration in the absence of NaSCN and multiplying by 100.
Enhanced resistance to systemic pneumococcal challenge in actively immunized adult and young mice.
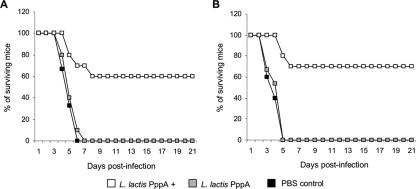
In order to determine whether the induction of specific antibodies by nasal immunization with L. lactis PppA+ correlated with increased resistance to pneumococcal infection, adult and young mice were intraperitoneally challenged with S. pneumoniae T14, and survival was closely monitored for 21 days. Adult mice immunized with L. lactis PppA+ showed a survival percentage of 60% on day 21 after challenge (Fig. 6A), while 100% of the PBS and L. lactis PppA adult mice died between days 4 and 7 after challenge. Nasal immunization of young mice with L. lactis PppA+ enabled 70% of the mice to survive up to day 21 postinfection (Fig. 6B), while 100% of the young mice in the control groups died on days 3 to 5 postchallenge. These results demonstrate that active immunization with L. lactis PppA+ increased resistance to the systemic challenge by S. pneumoniae in both groups.
FIG. 6.
Survival of adult (A) and young (B) mice after undergoing nasal immunization with recombinant L. lactis expressing the PppA protein on its surface (L. lactis PppA+) and intraperitoneal challenge with 108 cells of S. pneumoniae T14. Mice receiving noninduced L. lactis (L. lactis PppA) or PBS were used as controls.
Improved resistance to systemic pneumococcal challenge in passively immunized young mice.
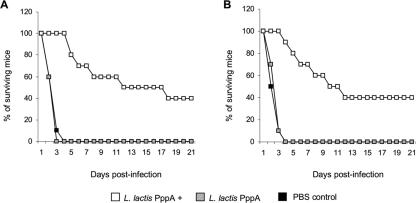
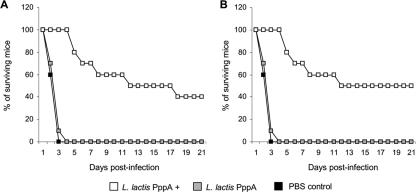
Protection against pneumococcal infection is considered to be antibody mediated. Therefore, we evaluated the ability of immune serum generated in L. lactis PppA+-immunized adult or young mice to provide protection for naïve young mice. In the first series of experiments, naïve young mice were passively immunized with the immune serum and challenged 24 h later. Passive immunization with pooled serum obtained from L. lactis PppA+ adult or young mouse groups significantly increased the survival percentage of the naïve young mice in relation to that of the respective control groups (Fig. 7A and B), with no significant differences between the young and adult groups (P = 0.91, Cox's F test). In a second series of experiments, naïve young mice were challenged with S. pneumoniae previously opsonized with the immune sera. Mice challenged with pneumococci opsonized with immune serum from L. lactis PppA+ adult mice showed a survival percentage of 40% (Fig. 8A), which was significantly higher than that of the control groups. When the pathogen was opsonized with the immune serum from L. lactis PppA+ of young mice, the survival percentage was 50% (Fig. 8B), a value significantly higher than the ones for the respective control groups but not significantly different from the values found in the mice that received the immune serum from the L. lactis PppA+ adult group (P = 0.64, Cox's F test).
FIG. 7.
Survival of naïve young mice passively immunized with pooled serum from adult (A) or young (B) mice nasally immunized with recombinant L. lactis expressing the PppA protein on its surface (L. lactis PppA+) and challenged 24 h later with 108 cells of S. pneumoniae T14. Naïve young mice receiving pooled sera from adult (A) or young (B) mice immunized with noninduced L. lactis (L. lactis PppA) or PBS were used as controls.
FIG. 8.
Survival of naïve young mice challenged with 108 cells of S. pneumoniae T14 suspended in PBS containing 10% of pooled sera from adult (A) or young (B) mice nasally immunized with recombinant L. lactis expressing the PppA protein on its surface (L. lactis PppA+). Naïve young mice challenged with S. pneumoniae opsonized with the pooled sera from adult (A) or young (B) mice immunized with noninduced L. lactis (L. lactis PppA) or PBS were used as controls.
Enhanced resistance of actively immunized adult and young mice to the respiratory challenge with different pneumococcal serotypes.
Since the pneumococcus bacterium is primarily a respiratory pathogen, we examined the ability of L. lactis PppA+ immunization to provide protection against a respiratory challenge. In addition, the ability of the recombinant L. lactis PppA+ to elicit cross-protection was evaluated by challenging adult and young mice with different pneumococcal serotypes. In control animals, all S. pneumoniae serotypes used were detected in lung cultures and hemocultures 2 days after the intranasal challenge. Nasal immunization with L. lactis PppA+ significantly reduced the lung bacterial cell counts of serotypes 3 and 6B (Table 4), while lung cultures were negative for serotypes 14 and 23F (Table 4) in both adult and young mice. Moreover, L. lactis PppA+ administration prevented the dissemination of all assayed pneumococcal serotypes, since hemocultures tested negative (Table 4).
TABLE 4.
Lung and blood bacterial cell counts after nasal challenge with different pneumococcal serotypesa
| Group | Response to serotype
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 3
|
6B
|
14
|
23F
|
|||||
| Lung (CFU/g) | Blood (CFU/ml) | Lung (CFU/g) | Blood (CFU/ml) | Lung (CFU/g) | Blood (CFU/ml) | Lung (CFU/g) | Blood (CFU/ml) | |
| Adult mice immunized with: | ||||||||
| L. lactis PppA+ | 3.8 ± 0.3** | <1.5** | 3.1 ± 0.2** | <1.5** | <1.5** | <1.5** | <1.5** | <1.5** |
| L. lactis PppA | 6.1 ± 0.4 | 4.2 ± 0.3 | 5.6 ± 0.5* | 3.9 ± 0.5 | 5.7 ± 0.4* | 4.1 ± 0.4 | 4.1 ± 0.3 | 3.6 ± 0.4 |
| Control | 6.9 ± 0.3 | 4.8 ± 0.2 | 6.3 ± 0.3 | 4.0 ± 0.2 | 6.1 ± 0.5 | 4.1 ± 0.7 | 4.3 ± 0.4 | 3.8 ± 0.5 |
| Young mice immunized with: | ||||||||
| L. lactis PppA+ | 4.2 ± 0.2** | <1.5** | 3.3 ± 0.5** | <1.5** | <1.5** | <1.5** | <1.5** | <1.5** |
| L. lactis PppA | 7.9 ± 0.4 | 4.6 ± 0.3 | 6.9 ± 0.3* | 4.4 ± 0.7 | 5.9 ± 0.6* | 4.3 ± 0.5 | 5.5 ± 0.5* | 4.1 ± 0.7 |
| Control | 8.3 ± 0.2 | 5.2 ± 0.2 | 7.5 ± 0.3 | 4.5 ± 0.3 | 6.9 ± 0.4 | 4.7 ± 0.4 | 6.2 ± 0.3 | 4.4 ± 0.2 |
Adult and young mice were immunized with recombinant L. lactis (pVE-PppA) with L. lactis PppA+ or without L. lactis PppA previous to induction with nisin. Results are expressed as log CFU/g of lung or log CFU/ml of blood. The lower limits of bacterial detection were 1.5 log CFU/g of lung and 1.5 log CFU/ml of blood. Asterisks represent significant differences from the respective control groups: *, P < 0.05; **, P < 0.01.
DISCUSSION
The PppA is a small S. pneumoniae protein that can be recovered in large quantities from the wash fluids of intact pneumococcal cells with PBS (15). PppA is antigenically conserved and exhibits a high level of sequence conservation in serotypes 3, 5, 9, 14, 19, and 23 (15). Although the role of PppA in the pathogenesis of pneumococcal infection is unknown, it has been demonstrated that antibodies against this antigen can reduce nasal colonization of pneumococci. However, the induction of specific anti-PppA antibodies by nasal immunization can be achieved only when the protein is administered with a mucosal adjuvant such as genetically modified cholera toxin (15). Cholera toxin is a potent adjuvant, but direct binding to and accumulation of this toxin in the nervous system following intranasal administration have been reported (13). As this is an important obstacle for the development of effective mucosal vaccines, the search for safe mucosal adjuvants becomes imperative. In this sense, LAB have been tested as potential adjuvants, since they are safe microorganisms, and some of them have been proved to have intrinsic immunoadjuvant properties when they are administered through the mucosal route (25, 33). Several authors have shown that live recombinant LAB can also elicit an efficient immune response, in which the strongest response was mounted when the antigen was bound to the membrane/cell wall compared to that of antigens that were secreted or that of intracellularly located antigens (4, 24, 27). In this work, we determined whether PppA anchored to the cell wall of a recombinant L. lactis strain was able to induce a protective immune response in mice of different ages.
The presence and levels of IgG and IgA pathogen-specific neutralizing antibodies are considered one of the most effective defense mechanisms against respiratory infections and a vital attribute of any vaccine designed to prevent these infections (32). In this work, detection of IgM, IgG, and IgA anti-PppA antibodies in the serum and BAL fluid of adult and young mice showed that nasal immunization with the recombinant L. lactis PppA+ strain was able to induce a strong mucosal and systemic immune response. The induced production of IgG- and IgA-specific PppA antibodies was significantly higher in young mice. Reviews of preclinical and human vaccine studies indicate that although early life immunization may not lead to early and strong antibody responses, it may result in efficient immunological priming, which can serve as an excellent basis for future responses (3, 30). Our results also confirmed the adjuvant properties of L. lactis NZ9000. Moreover, M. Medina, J. Villena, S. Salva, E. Vintiñi, P. Langella, and S. Alvarez (submitted for publication) have shown that nasal administration of nonrecombinant L. lactis NZ9000, at the appropriate dose, enhanced the activity of alveolar and peritoneal phagocytes in mouse. This agrees with recent findings that demonstrated that L. lactis NZ9000 cells are able to stimulate in vitro the production of tumor necrosis factor-α by murine peritoneal macrophages and to upregulate the expression of costimulatory molecules in bone marrow-derived dendritic cells (2). Thus, the stimulation of antigen-presenting cells by L. lactis would be able to induce the development of a stronger specific immune response when administered together with the PppA antigen. In addition, it is important to note that no antibodies against L. lactis were found in the serum or the BAL fluid of adult and young mice immunized with the recombinant strain (data not shown). Thus, the host immune response was directed against the protein expressed by L. lactis and not against the vector.
We found that both the IgG1 and the IgG2a anti-PppA antibodies were produced. Although data for the role of different IgG subclasses in the protection against pneumococcal infection are limited, it seems that the IgG2a subclass is particularly important, since it is the primary murine IgG subclass that mediates optimal complement fixation (21). In addition, although IgG1 is not efficient at complement fixation, it might also contribute to protection against pneumococcal infection through Fc receptor binding or by preventing attachment and colonization of bacteria at mucosal surfaces. Thus, the production of both types of antibodies would increase resistance against infection. Adult and young mice immunized with L. lactis PppA+ showed significantly higher survival percentages than the control groups after systemic challenge with S. pneumoniae, which would indicate that anti-PppA antibodies would be able to increase resistance against the pneumococcal invasive disease. The survival of naïve young mice receiving L. lactis PppA+ immune serum confirmed the direct correlation between anti-PppA antibodies and protection.
Capsule-specific antibodies have been shown to be highly protective; however, the exact concentration of these serotype-specific antibodies required for protection against disease is still uncertain. Moreover, it is clear that the opsonic activity and avidity of these antibodies are more critical determinants of protection than their concentration, especially in young individuals (5, 23, 31). These findings could also be applied to the antibodies directed against protein antigens on the surface of S. pneumoniae. In the present work, the avidity of anti-PppA antibodies elicited in young mice was similar to that found in the adult group. However, in order to understand the mechanisms of antibody action in each group, it would be necessary to perform a comparative analysis of the opsonic activity of the immune sera of adult and young mice; consequently it merits further investigation.
Challenge assays with pneumococcal serotypes 3, 6B, 14 and 23F were performed to evaluate the efficacy of immunization with L. lactis PppA+ against respiratory infection produced with different serotypes of S. pneumoniae,. These serotypes were selected according to previous epidemiological studies that determined serotype 14 was the most prevalent serotype in our country (22) and also because serotypes 3, 6B, 9, 14, 18, 19y, and 23F are the serotypes most often associated with invasive disease (17). As expected, young mice were more susceptible to respiratory infection than adult mice. However, immunization of young and adult mice with L. lactis PppA+ provided protection against the serotypes assessed, although to a higher degree in the latter group. This fact could indicate that vaccination at an early age would be a better strategy for improved protection of the host against S. pneumoniae infection. It seems likely that the BAL fluid anti-PppA antibodies, which were found in L. lactis PppA+-immunized young mice at higher levels than in the adult group, were involved in this effect.
In summary, the results presented in this study showed that nasal immunization with PppA anchored to the cell wall of a recombinant L. lactis strain elicited cross-protective immunity against different pneumococcal serotypes, afforded protection against both systemic and respiratory challenges, and induced protective immunity in young and adult mice. Our results also suggest that immunization with the recombinant L. lactis strain is more effective when the vaccination begins at an early age (young mice).
Acknowledgments
This work was supported by grants from FONCyT PICT 99, no. 5-7206, CIUNT 26 D/202, and CIUNT D/305, CONICET Res. 1257/4.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 7 April 2008.
REFERENCES
- 1.Anttila, M., J. Eskola, H. Åhman, and H. Käyhty. 1998. Avidity of IgG for Streptococcus pneumoniae type 6B and 23F polysaccharides in infants primed with pneumococcal conjugates and boosted with polysaccharide or conjugate vaccines. J. Infect. Dis. 1771614-1621. [DOI] [PubMed] [Google Scholar]
- 2.Audouy, S. A. L., M. L. van Roosmalen, J. Neef, R. Kanninga, E. Post, M. van Deemter, H. Metselaar, S. van Selm, G. T. Robillard, K. J. Leenhouts, and P. W. M. Hermans. 2006. Lactococcus lactis GEM particles displaying pneumococcal antigens induce local and systemic immune responses following intranasal immunization. Vaccine 245434-5441. [DOI] [PubMed] [Google Scholar]
- 3.Barrios, C., P. Brawand, M. Berney, C. Brandt, P. H. Lambert, and C. A. Siegrist. 1996. Neonatal and early life immune responses to various forms of vaccine antigens qualitatively differ from adult responses: predominance of a Th2-biased pattern which persists after adult boosting. Eur. J. Immunol. 261489-1496. [DOI] [PubMed] [Google Scholar]
- 4.Bermudez-Humarán, L. G., N. G. Cortes-Perez, Y. Le Loir, J. M. Alcocer-González, R. S. Tamez-Guerra, R. Montes de Oca-Luna, and P. Langella. 2004. An inducible surface presentation system improves cellular immunity against human papillomavirus type 16 E7 antigen in mice after nasal administration with recombinant lactococci. J. Med. Microbiol. 53427-433. [DOI] [PubMed] [Google Scholar]
- 5.Bernatoniene, J., and A. Finn. 2005. Advances in pneumococcal vaccines: advantages for infants and children. Drugs 65229-255. [DOI] [PubMed] [Google Scholar]
- 6.Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. Hansen, L. Elvin, K. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, and K. Edwards. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 19187-195. [DOI] [PubMed] [Google Scholar]
- 7.Bogaert, D., P. W. M. Hermans, P. V. Adrian, and R. Groot. 2004. Pneumococcal vaccines: an update on current strategies. Vaccine 222209-2220. [DOI] [PubMed] [Google Scholar]
- 8.Bogaert, D., R. Groot, and P. W. M. Hermans. 2004. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 4144-154. [DOI] [PubMed] [Google Scholar]
- 9.Briles, D. E., R. C. Tart, E. Swiatlo, J. P. Dillard, P. Smith, K. A. Benton, B. A. Ralph, A. Brooks-Walter, M. J. Crain, S. K. Hollingshead, and L. S. McDaniel. 1998. Pneumococcal diversity: considerations for new vaccine strategies with emphasis on pneumococcal surface protein A (PspA). Clin. Microbiol. Rev. 11645-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buccato, S., D. Maione, C. D. Rinaudo, G. Volpini, A. R. Taddei, R. Rosini, J. L. Telford, G. Grandi, and I. Margarit. 2006. Use of Lactococcus lactis expressing pili from group B Streptococcus as a broad-coverage vaccine against streptococcal disease. J. Infect. Dis. 194331-340. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. 2000. Preventing pneumococcal disease among infants and young children. Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb. Mortal. Wkly. Rep. 491-38.10993565 [Google Scholar]
- 12.Cortes-Perez, N. G., L. G. Bermudez-Humaran, Y. Le Loir, C. Rodriguez-Padilla, A. Gruss, O. Saucedo-Cardenas, P. Langella, and R. Montes-de-Oca-Luna. 2003. Mice immunization with live lactococci displaying a surface anchored HPV-16 E7 oncoprotein. FEMS Microbiol. Lett. 22937-42. [DOI] [PubMed] [Google Scholar]
- 13.Fujihashi, K., T. Koga, F. W. van Ginkel, Y. Hagiwara, and J. R. McGhee. 2002. A dilemma for mucosal vaccination: efficacy versus toxicity using enterotoxin-based adjuvants. Vaccine 202431-2438. [DOI] [PubMed] [Google Scholar]
- 14.Gor, D. O., X. Ding, D. E. Briles, M. R. Jacobs, and N. S. Greenspan. 2005. Relationship between surface accessibility for PpmA, PsaA, and PspA and antibody-mediated immunity to systemic infection by Streptococcus pneumoniae. Infect. Immun. 731304-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green, B. A., Y. Zhang, A. W. Masi, V. Barniak, M. Wetherell, R. P. Smith, M. S. Reddy, and D. Zhu. 2005. PppA, a surface-exposed protein of Streptococcus pneumoniae, elicits cross-reactive antibodies that reduce colonization in a murine intranasal immunization and challenge model. Infect. Immun. 73981-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanniffy, S. B., A. T. Carter, E. Hitchin, and J. M. Wells. 2007. Mucosal delivery of a pneumococcal vaccine using Lactococcus lactis affords protection against respiratory infection. J. Infect. Dis. 195185-193. [DOI] [PubMed] [Google Scholar]
- 17.Hausdorff, W. P., D. R. Feikin, and K. P. Klugman. 2005. Epidemiological differences among pneumococcal serotypes. Lancet Infect. Dis. 583-93. [DOI] [PubMed] [Google Scholar]
- 18.Hebert, H. M., R. Raya, and G. S. de Giori. 2000. Nutritional requirements and nitrogen-dependent regulation of proteinase activity of Lactobacillus helveticus CRL 1062. Appl. Environ. Microbiol. 665316-5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson, C. R. 2002. Clinical experience with pneumococcal conjugate vaccines in infants and children. J. Am. Osteopath. Assoc. 102431-436. [PubMed] [Google Scholar]
- 20.Le Loir, Y., V. Azevedo, S. C. Oliveira, D. A. Freitas, A. Miyoshi, L. G. Bermudez-Humaran, S. Nouaille, L. A. Ribeiro, S. Leclercq, J. E. Gabriel, V. D. Guimaraes, M. N. Oliveira, C. Charlier, M. Gautier, and P. Langella. 2005. Protein secretion in Lactococcus lactis: an efficient way to increase the overall heterologous protein production. Microb. Cell Fact. 42-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mawas, F., I. M. Feavers, and M. J. Corbel. 2001. Serotype of Streptococcus pneumoniae capsular polysaccharide can modify the Th1:Th2 cytokine profile and IgG subclass response to pneumococcal-CRM197 conjugate vaccines in a murine model. Vaccine 191159-1166. [DOI] [PubMed] [Google Scholar]
- 22.Mollerach, M., M. Regueira, L. Bonofiglio, R. Callejo, J. Pace, J. L. Di Fabio, S. Hollingshead, D. Briles, and the Streptococcus pneumoniae Working Group. 2004. Invasive Streptococcus pneumoniae isolates from Argentinian children: serotypes, families of pneumococcal surface protein A (PspA) and genetic diversity. Epidemiol. Infect. 132177-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Musher, D. M., A. J. Chapman, A. Goree, S. Jonsson, D. Briles, and R. E. Baughn. 1986. Natural and vaccine-related immunity to Streptococcus pneumoniae. J. Infect. Dis. 154245-256. [DOI] [PubMed] [Google Scholar]
- 24.Norton, P. M., H. W. Brown, J. M. Wells, A. M. Macpherson, P. W. Wilson, and R. W. Le Page. 1996. Factors affecting the immunogenicity of tetanus toxin fragment C expressed in Lactococcus lactis. FEMS Immunol. Med. Microbiol. 14167-177. [DOI] [PubMed] [Google Scholar]
- 25.Racedo, S., J. Villena, M. Medina, G. Agüero, V. Rodríguez, and S. Alvarez. 2006. Lactobacillus casei administration reduces lung injuries in a Streptococcus pneumoniae infection. Microbes Infect. 82359-2366. [DOI] [PubMed] [Google Scholar]
- 26.Reveneau, N., M. C. Geoffroy, C. Locht, P. Chagnaud, and A. Mercenier. 2002. Comparison of the immune responses induced by local immunizations with recombinant Lactobacillus plantarum producing tetanus toxin fragment C in different cellular locations. Vaccine 201769-1777. [DOI] [PubMed] [Google Scholar]
- 27.Robinson, K., L. M. Chamberlain, M. C. Lopez, C. M. Rush, H. Marcotte, R. W. F. Le Page, and J. M. Wells. 2004. Mucosal and cellular immune responses elicited by recombinant Lactococcus lactis strains expressing tetanus toxin fragment C. Infect. Immun. 722753-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook, J., E. F. Fritch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 29.Sarno Oliveira, M. L., A. P. Mattos Areas, I. Barros Campos, V. Monedero, G. Perez-Martines, E. M. Miyaji, L. C. Cerqueira Leite, A. K. Araujo, and P. Lee Ho. 2006. Induction of systemic and mucosal immune response and decrease in Streptococcus pneumoniae colonization by nasal inoculation of mice with recombinant lactic acid bacteria expressing pneumococcal surface antigen A. Microbes Infect. 81016-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siegrist, C. A. 2001. Neonatal and early life vaccinology. Vaccine 193331-3346. [DOI] [PubMed] [Google Scholar]
- 31.Tai, S. S. 2006. Streptococcus pneumoniae protein vaccine candidates: properties, activities and animal studies. Crit. Rev. Microbiol. 32139-153. [DOI] [PubMed] [Google Scholar]
- 32.Twigg, H. L. 2005. Humoral immune defense (antibodies): recent advances. Proc. Am. Thorac. Soc. 2417-421. [DOI] [PubMed] [Google Scholar]
- 33.Villena, J., S. Racedo, G. Agüero, E. Bru, M. Medina, and S. Alvarez. 2005. Lactobacillus casei improves resistance to pneumococcal respiratory infection in malnourished mice. J. Nutr. 1351462-1469. [DOI] [PubMed] [Google Scholar]