Abstract
Adenomatous polyposis coli (APC) mutations are linked to human and mouse colorectal cancers. The Apc multiple intestinal neoplasia (Min) mouse mutation causes adenomas to develop throughout the small and large intestines. The BALB-Min (C.B6-ApcMin/+) congenic strain was generated by backcrossing into BALB/c the ApcMin allele from C57BL/6J-ApcMin/+ mice. BALB-Min mice have a low tumor multiplicity (27.4 small intestine tumors/mouse) and a relatively long life span (>1 year) that makes them amenable to long-term studies. To investigate the interplay of the adaptive immune system and intestinal tumorigenesis, the immunodeficient compound mutant strain BALB-RagMin (C.Cg-Rag2−/− ApcMin/+) was generated. BALB-RagMin mice had a significant increase in tumors in the small, but not large, intestine relative to their BALB-Min counterparts (43.0 versus 24.0 tumors/mouse, respectively). The results suggest that the adaptive immune system plays a role in either the elimination or the equilibrium phase of cancer immunoediting in the small intestine in this model. We investigated the effect of the enterohepatic bacterial pathogen Helicobacter hepaticus on liver and intestine tumorigenesis in BALB-RagMin mice. H. hepaticus-infected BALB-RagMin mice developed moderate hepatitis, moderate typhlitis, and mild colitis. There were no differences in small intestine and cecal tumor multiplicity, regionality, or size relative to that in uninfected mice. However, H. hepaticus-infected BALB-RagMin mice had a significant increase in colon tumor incidence relative to uninfected BALB-RagMin mice (23.5% versus 1.7%, respectively). The data suggest that H. hepaticus, which is present in many research colonies, promotes colon tumorigenesis in the BALB-RagMin mouse and that it has the potential to confound colon tumorigenesis studies.
Colorectal cancer (CRC) is the third most commonly diagnosed cancer in both men and women in the United States and is responsible for 10% of all cancer deaths (1). Mutations in the tumor suppressor gene APC (adenomatous polyposis coli) have been identified in >85% of patients with familial adenomatous polyposis (21, 28) and in >80% of sporadic forms of CRC (29, 48). Therefore, APC is one of the key genes in the pathogenesis of CRC. APC participates in the WNT-β-catenin signaling pathway. Loss of functional APC results in the stabilization of β-catenin in the cytoplasm and its subsequent translocation into the nucleus. In the nucleus, β-catenin binds to and activates members of the TCF/LEF family of transcription factors, leading to the activation of their target genes, including c-MYC, CCND1 (cyclin D-1), and MMP7 (31, 40, 53).
The C57BL/6J-ApcMin/+ (B6-Min [multiple intestinal neoplasia]) mouse strain harbors an N-ethyl-N-nitrosourea-induced, dominant nonsense mutation at codon 850 in the mouse homolog of APC (42, 43, 59). Therefore, B6-Min is a clinically relevant mouse model for studying the pathogenesis, prevention, and treatment of human CRC. Homozygous B6-Min embryos die in utero due to a defect in epiblast cell development (25, 44). Heterozygous B6-Min mice appear grossly normal but develop numerous sessile or polypoid adenomas in the intestine, the vast majority occurring in the small intestine (42). Conditional Apc mouse mutants have been used to show that APC is required for the normal development of many epithelial derivatives, including skin and thymus (20, 33). Unfortunately, B6-Min mice rarely survive beyond 150 days, death typically resulting from complications of the large tumor load, either chronic blood loss or intestinal blockage (58). This short life span precludes long-term studies.
Genetic studies suggest that multiple genes regulate tumor multiplicity in the B6-Min mouse (9, 52, 58, 62). The modifier of Min1 (Mom1) locus contributes to ∼50% of the genetic variation in tumor multiplicity and size (9, 19). Breeding studies have shown that B6/J, BTBR/Pas, and 129Sv/Pas strains harbor a susceptible Mom1S locus that results in high tumor loads. In contrast, AKR/J, BALB/cByJ, CAST/EiJ, C3H/HeJ, DBA/2J, MA/MyJ, and SWR/J strains harbor a resistant Mom1R locus. The introduction of the ApcMin allele into these strains results in low tumor multiplicities and a concomitant longer life span (9, 19, 30, 41). We backcrossed the ApcMin allele from B6-Min into the BALB/c strain, generating the BALB-Min strain (C.B6-ApcMin/+). We document tumor multiplicity, tumor regionality, and life span in BALB-Min mice.
B6-Min mice homozygous for either severe combined immunodeficiency (SCID; Prkdcscid) or beige (Lystbg−J) mutations had tumor multiplicities and regional distributions that were equivalent to those of immunocompetent B6-Min mice (10, 11), suggesting that the immune system has minimal or no effect on tumorigenesis in this model.
Recombination activating genes 1 and 2 (Rag1 and Rag2) are required for T-cell receptor and immunoglobulin gene rearrangements that lead to the generation of functional T and B lymphocytes (2). Rag1- or Rag2-null mice show a nonleaky SCID phenotype characterized by the absence of mature T and B cells (39, 56). In contrast to the Prkdcscid or Lystbg−J data, it was recently reported that B6-Rag2−/− ApcMin/+ mice develop significantly more small intestine tumors than B6-Min mice (49). We introduced the Rag2−/− allele into BALB-Min to further investigate the relationship of the adaptive immune system with Min intestinal tumorigenesis and to characterize intestinal tumorigenesis in Rag2−/− ApcMin/+ mice on a Mom1R background.
Helicobacter hepaticus is a gram-negative, urease-producing, spiral-shaped bacterium that is a common pathogen in laboratory mouse colonies (17, 60). Enterohepatic Helicobacter species, including H. hepaticus, are found predominantly in the cecum and colon but can also infect the liver in susceptible mice. H. hepaticus induces chronic active hepatitis and hepatocellular neoplasms in susceptible mouse strains (18, 61), typhlocolitis in C57BL/10 interleukin-10 (IL-10)-null mice (6, 32), and typhlocolitis and colitis-associated adenocarcinomas in 129/SvEv-Rag2−/− mice (13). We recently reported that H. hepaticus promotes azoxymethane-initiated colon tumor development in BALB/c-IL-10-deficient mice (45). Here, we investigated whether H. hepaticus infection promotes intestinal tumorigenesis in the BALB-RagMin mouse model.
We report that the BALB-Min strain has a reduced intestinal tumor multiplicity and a concomitant long life span allowing for long-term studies, that BALB-RagMin mice have a significant increase in small intestine but not large intestine tumors, and that H. hepaticus infection of BALB-RagMin mice promotes colon, but not small intestine, tumorigenesis.
MATERIALS AND METHODS
Mouse strains and husbandry.
C57BL/6J-ApcMin/+ (B6-Min) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained by backcrossing to C57BL/6J females at the MIT Division of Comparative Medicine. Progeny at each generation were genotyped for the ApcMin allele by using a PCR assay (35). BALB-Rag2−/− mice [C.129S6(B6)-Rag2tm1Fwa] were purchased from Taconic Farms, Inc. (Hudson, NY) and maintained as a closed colony.
The BALB-Min (C.B6-ApcMin/+) strain was generated by backcrossing the ApcMin allele from B6-Min mice into the BALB/cJ strain (The Jackson Laboratory; Bar Harbor, ME) for the first six generations and then into BALB/cAnNTac (Taconic Farms, Inc., Hudson, NY) for the next six generations. The BALB-Min strain was maintained as a closed colony by mating BALB-Min males to wild-type BALB Apc+/+ females from the colony and genotyping by PCR for the ApcMin allele.
BALB-RagMin (C.Cg-Rag2−/− ApcMin/+) double-mutant mice were generated by crossing BALB-Rag2−/− and BALB-Min mice and identifying ApcMin progeny by PCR. BALB-Rag2+/− ApcMin/+ progeny were backcrossed to BALB-Rag2−/− mice and BALB-Rag2−/− ApcMin/+ progeny identified by PCR for Rag2−/− and ApcMin alleles. The Rag2 PCR assay was based on primer sequences provided by Taconic Biotechnology (RagA, 5′-GGGAGGACACTCACTTGCCAG-3′; RagB, 5′-AGTCAGGAGTCTCCATCTCAC-3′; and NeoA, 5′-CGGCCGGAGAACCTGCGTGCAA-3′) and resulted in amplified product sizes of 263 bp for the wild-type allele and 350 bp for the null allele.
Mice were provided LabDiet RMH 3000 (PMI Nutrition International, LLC, Brentwood, MO) and filtered water ad libitum. All experiments were approved by the MIT Committee on Animal Care and performed in accordance with the Guide for the Care and Use of Laboratory Animals (47) in a facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, International. The colony was routinely monitored for adventitious viral, bacterial, and parasitic pathogens by a sentinel program and was found to be free of the following agents: mouse hepatitis virus, mouse rotavirus (EDIM), mouse parvovirus, minute virus of mice, ectromelia virus, Sendai virus, pneumonia virus of mice, respiratory enteric virus III (Reo3), encephalomyelitis virus (GD7), lymphocytic choriomeningitis virus, K-virus, polyoma virus, mouse adenovirus, Mycoplasma pulmonis, cilium-associated respiratory bacillus, Salmonella spp., Citrobacter rodentium, Helicobacter spp., Pasteurella spp., Corynebacterium kutscherii, Bordetella bronchiseptica, Pseudomonas aeruginosa, Klebsiella spp., beta-hemolytic Streptococcus spp., Streptococcus pneumoniae, and endo- and ectoparasites.
H. hepaticus culture, inoculation, and PCR.
H. hepaticus strain 3B1 (ATCC 51449) was cultured on tryptic soy agar (BD, Franklin Lakes, NJ) supplemented with 5% defibrinated sheep red blood cells (Quad Five, Ryegate, MT) at 37°C under microaerobic conditions (80% N2, 10% CO2, 10% H2) (17). For experimental inoculation studies, the bacteria were grown overnight in brucella broth (BD, Franklin Lakes, NJ) supplemented with 5% heat-inactivated fetal bovine serum; examined for purity, viability, and motility at ×400 magnification with dark-field illumination; pelleted; and then resuspended to an optical density at 600 nm of 1.0 (∼108 organisms/ml). Fifty-eight BALB-RagMin mice (20 males and 38 females, 5 to 10 weeks of age) were gavaged once with 0.2 ml of bacterial suspension (∼2 × 108 organisms). H. hepaticus-inoculated BALB-RagMin mice were euthanized at 12 to 13 weeks postinoculation (wpi) or 23 to 24 wpi or if they developed opportunistic infections. Sixty untreated BALB-RagMin mice (26 male and 34 female) of comparable ages served as controls.
To confirm H. hepaticus infection, fecal pellets were collected at 2 wpi and/or at necropsy from each mouse. Fecal DNAs were prepared for individual mice by using a QIAamp DNA stool kit (Qiagen, Inc., Valencia, CA). Five microliters of the fecal DNA was used in a 25-μl all-Helicobacter and all-Lactobacillus duplex PCR assay, using PuReTaq ready-to-go beads (GE Healthcare, Chalfont St. Giles, United Kingdom), all-Helicobacter primers (C97 and C05; amplicon size = 1.2 kb) (16), and all-Lactobacillus primers (Lac1 and Lac2; amplicon size = 340 bp) (5). PCR amplification was for 35 cycles, each cycle consisting of 1 min at 94°C, 2.5 min at 58°C, and 3 min at 72°C, with a final, 7-min extension at 72°C. A 10-μl aliquot of the PCR was fractionated on 1% agarose gels containing 0.03% ethidium bromide in Tris-acetate-EDTA buffer and visualized on a Kodak Image Station 1000 (PerkinElmer Life Science, Boston, MA).
Postmortem examination, histopathology, and immunohistochemistry (IHC).
Mice were euthanized by CO2 inhalation. The small and large intestines were removed and flushed with phosphate-buffered saline (PBS) by using a 10-ml syringe and a 24-gauge gavage needle (FST, Inc., Foster City, CA). The colon was separated from the cecum and opened longitudinally on PBS-moistened bibulous paper (VWR Scientific Products, West Chester, PA). Remaining fecal material was removed with a stream of PBS from a squirt bottle. The colon was fixed flat between bibulous papers in 10% neutral-buffered formalin and then stored in 70% ethanol. The small intestine was treated similarly except that it was divided into four equal segments, with proximal and distal orientations being noted for each segment. The cecum was flushed with PBS, spread flat on a foam biopsy pad (Surgipath Medical Industries, Inc., Richmond, IL), placed in a histology cassette, and fixed in 10% neutral-buffered formalin.
Tumors were enumerated under a dissecting microscope at ×10 magnification for the entireties of the small and large intestines. Tumor size was taken at a tumor's greatest width and was measured to the nearest 0.1 mm with the aid of an eyepiece reticle. The lengths of the proximal, middle, and distal thirds of the small intestine were calculated by summing the lengths of the four segments and dividing by 3. The same person (C. M. Nagamine) performed all tumor counts and measurements.
For histological analyses, small intestines and colons were processed as “Swiss rolls” (4). Livers, small intestines, ceca, and/or colons were embedded in Paraplast Extra (VWR Scientific Products, West Chester, PA), sectioned at 4 μm, and stained with hematoxylin and eosin. Representative slides of the liver were scored in a blinded fashion by a board-certified veterinary pathologist (A. B. Rogers) for severity of lobular (0 to 4), periportal (0 to 4), and interface (0 to 4) inflammation and presence of lesions in one or multiple liver lobes (0 to 4). The sum of the individual scores (maximum = 16) was designated the liver disease index. Hepatitis was defined as a liver disease index of >4.0. Representative slides of the cecum and colon were scored in a blinded fashion by a board-certified veterinary pathologist (B. Rickman) for inflammation (0 to 4) and hyperplasia (0 to 4). The sum of the individual scores for a cecum or colon sample (maximum = 8) was designated the cecal or colon disease index, respectively.
Paraffin-embedded small intestine, cecum, and/or colon samples from two to six mice representing each of the experimental and control groups were subjected to IHC for Ki67, a cell proliferation-associated nuclear antigen (54), and β-catenin, a cytoplasmic protein that is associated with adherens junctions and APC. Absence of functional APC proteins results in robust β-catenin immunostaining of the cytoplasm and nucleus, in addition to its normal localization on the cell membrane (24, 55).
IHC for Ki67-reactive cells was performed on sections following heat-induced epitope retrieval in target retrieval solution, pH 6 (Dako, Inc., Carpinteria, CA). Mouse monoclonal antibody reactive against human Ki67 protein (1:50 dilution; BD Biosciences, San Diego, CA) was complexed with biotinylated anti-mouse immunoglobulin by using an animal research kit (Dako, Inc., Carpinteria, CA). Incubation of the antibody complexes, incubation with streptavidin-horseradish peroxidase conjugates, and visualization with 3,3′-diaminobenzidine (DAB) were performed according to the manufacturer's recommendations. IHC for β-catenin was performed using rabbit anti-β-catenin 1° antibody (1:100 dilution; NeoMarkers, Inc., Fremont, CA), biotinylated goat anti-rabbit 2° antibody (1:1,000 dilution; Dako, Inc., Carpinteria, CA), streptavidin-horseradish peroxidase, and DAB. IHC slides were counterstained with hematoxylin. Positive and negative controls (staining with an irrelevant species-, isotype-, and concentration-matched 1° antibody) were performed with each IHC assay.
Statistical analysis.
Statistical analyses comparing tumor characteristics and colon and liver indices were performed with InStat 3 (version 3.0b; GraphPad Software, Inc., San Diego, CA).
RESULTS
Low tumor multiplicity and long life span in BALB-Min mice.
B6-Min mice in our colony had a 100% prevalence of small intestine tumors and a median small intestine tumor multiplicity of 76.5 tumors/mouse at 4 to 6 months of age (Table 1). The prevalences of cecal and colon tumors were 66.7% (12/18) and 77.8% (14/18), respectively. B6-Min mice in our colony rarely lived longer than 6 months. The BALB-Min strain was generated to circumvent the short life span inherent in B6-Min. Although the prevalence of small intestine tumors in BALB-Min mice was also 100%, the median small intestine tumor multiplicity was 24.0 tumors/mouse at 5 to 15 months of age, significantly lower than that in B6-Min mice (Table 1) (P < 0.001; Mann-Whitney test). Cecal and colon tumor prevalences were minimal, with only 1/17 (5.9%) mice having cecal or colon tumors. The low tumor multiplicity resulted in a concomitant increase in life span, with some mice living >1 year.
TABLE 1.
Small intestine tumor multiplicity and prevalence of cecal and colon tumors in B6-Min, BALB-Min, BALB-RagMin, and H. hepaticus-infected BALB-RagMin micea
| Strain | No. of mice | Age range (mo) | SI tumor multiplicity mean ± SD (median) | % of mice with tumors (no. with tumors/total no.)
|
|
|---|---|---|---|---|---|
| Cecal | Colon | ||||
| B6-Min | 18 | 4-6 | 101.1 ± 58.5 (76.5)A | 66.7 (12/18)D | 77.8 (14/18)E |
| BALB-Min | 17 | 5-15 | 27.4 ± 13.9 (24.0)A,B,C | 5.9 (1/17)D | 5.9 (1/17)E |
| BALB-RagMin | 60 | 4-9 | 46.0 ± 29.0 (43.0)B | 0 (0/60) | 1.7 (1/60)F |
| BALB-RagMin (with H. hepaticus infection) | 34 | 4-8 | 38.5 ± 16.6 (37.5)C | 5.9 (2/34) | 23.5 (8/34)F |
The Mann-Whitney test was used to determine if differences in small intestine (SI) tumor multiplicity were significant. Fisher's exact test was used to determine if differences in cecal and colon tumor prevalence were significant. Values with the same superscript are significantly different from each other. A, E, and F, P ≤ 0.001; B and D, P < 0.01; C, P < 0.05.
Tumor regionality in the small intestine was determined by enumerating the tumors for the proximal, middle, and distal thirds of the small intestine. In B6-Min mice, most of the tumors were observed in the distal third of the small intestine (Table 2). Other investigators have reported similar results (57). In BALB-Min mice, more tumors were found in the middle third of the small intestine although this trend did not reach statistical significance (Table 2).
TABLE 2.
Small intestine tumor regionality in B6-Min, BALB-Min, and BALB-RagMin micea
| Strain | No. of mice | Age range (mo) | Mean ± SD (median) for SI region
|
||
|---|---|---|---|---|---|
| Proximal | Middle | Distal | |||
| B6-Min | 18 | 4-6 | 10.5 ± 5.2 (9.5)A,B | 36.1 ± 22.1 (28.0)A | 54.5 ± 32.7 (46.0)B |
| BALB-Min | 17 | 5-15 | 7.9 ± 4.3 (6.0) | 11.5 ± 7.9 (9.0) | 7.9 ± 6.2 (8.0) |
| BALB-RagMin | 60 | 4-9 | 14.0 ± 8.8 (12.5)C | 19.9 ± 13.6 (18.0)C,D | 12.2 ± 9.5 (11.0)D |
| BALB-RagMin with H. hepaticus | 34 | 4-8 | 10.5 ± 5.3 (10.0)E | 16.8 ± 8.5 (15.5)E,F | 11.1 ± 6.8 (11.0)F |
Values with the same superscript were significantly different by Dunn's multiple-comparison test. A, B, and D, P < 0.001; E, P < 0.01; C and F, P < 0.05. SI, small intestine.
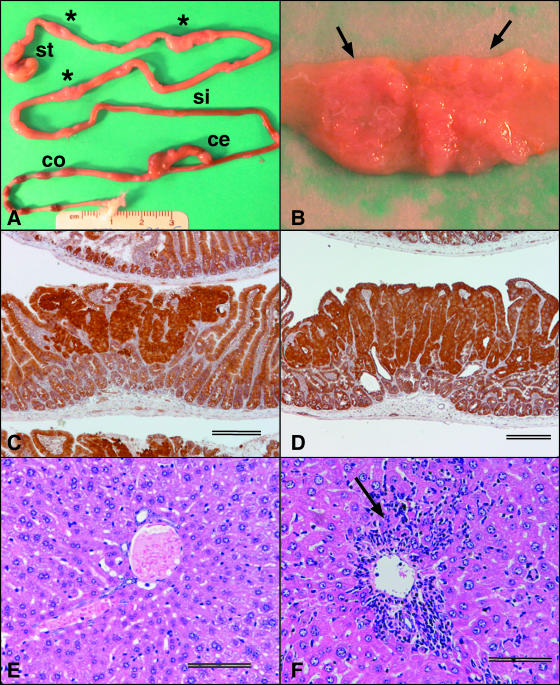
BALB-Min small intestine tumors were sufficiently large in older mice that they resulted in easily visible dilatations of the intestinal wall at necropsy (Fig. 1A). The tumors were sessile and grew laterally (Fig. 1B). Because median tumor size increased with age (data not shown), we determined tumor sizes for the proximal, middle, and distal regions of the small intestine for four B6-Min mice and four BALB-Min mice at the same age (5 months). Median tumor size was largest in the proximal region of the small intestine for both B6-Min and BALB-Min mice (Table 3) (2.5 mm and 2.9 mm, respectively) and smallest in the distal region of the small intestine (2.1 mm and 1.5 mm, respectively). Although median tumor size did not differ between the two strains in the proximal region of the small intestine (P = 0.431; Mann-Whitney test), BALB-Min mice had a larger median tumor size in the middle region (2.4 mm, versus 2.0 mm for B6-Min [P < 0.05; Mann-Whitney test]) but a smaller median tumor size in the distal region (1.5 mm, versus 2.1 mm for B6-Min [P < 0.01; Mann-Whitney test]) of the small intestine. The data show that despite a lower tumor multiplicity in BALB-Min mice, median tumor size was not necessarily smaller except in the distal region of the small intestine.
FIG. 1.
(A) Whole mount of gastrointestinal tract of an 11-month-old BALB-Min mouse. Asterisks identify dilatations in the small intestine. st, stomach; si, small intestine; ce, cecum; co, colon. (B) Mucosal surface of one of the dilatations in panel A, showing the presence of large, sessile adenomas (arrows). (C) Photomicrograph of BALB-Min small intestine adenoma showing IHC staining for β-catenin. Scale bar = 250 μm. (D) Photomicrograph of BALB-RagMin small intestine adenoma showing IHC staining for β-catenin. The IHC staining pattern is similar to that for BALB-Min (C). Scale bar = 250 μm. (E) Photomicrograph of liver of uninfected female, 7 months of age, BALB-RagMin. The liver disease index was 1.0/16.0. Scale bar = 100 μm. (F) Liver of female BALB-RagMin mouse at 24 wpi (7 months of age) with periportal hepatitis (arrow). The liver disease index was 10.5/16.0. Scale bar = 100 μm.
TABLE 3.
Median tumor size in the proximal, middle, and distal small intestine regions of B6-Min, BALB-Min, and BALB-RagMin micea
| Strain | No. of mice | Mean age in mo (range) | Median (range) (mm) for SI region
|
||
|---|---|---|---|---|---|
| Proximal | Middle | Distal | |||
| B6-Min | 4 | 5 | 2.5 (0.5-5.9)A | 2.0 (0.4-4.1)1 | 2.1 (0.7-3.6)A,2 |
| BALB-Min | 4 | 5 (4.9-5.4) | 2.9 (1.0-7.0)B | 2.4 (0.8-5.1)1,3 | 1.5 (0.5-3.0)B,2 |
| BALB-RagMin | 8 | 5 (4.9-5.4) | 2.3 (0.5-5.5)C | 2.0 (0.5-6.0)3 | 1.2 (0.4-2.6)C |
Median tumor size is largest in the proximal region of the small intestine (SI) and smallest in the distal region of the small intestine in all strains. Values with the same superscript (letters for comparisons within a row and numerals for comparisons within a column) are statistically significant by the Mann-Whitney test. B, C, and 3, P < 0.001; A and 2, P < 0.01; 1, P < 0.05.
BALB-RagMin mice have an increase in small intestine tumor multiplicity.
To investigate the effect of the adaptive immune system on tumorigenesis, we introduced the Rag2-null allele into BALB-Min. Immunodeficient BALB-RagMin mice had a significant increase in small intestine tumors relative to the parental BALB-Min strain (Table 1) (P < 0.01; Mann-Whitney test). The median small intestine tumor multiplicity in BALB-RagMin mice was 43.0 tumors/mouse, versus 24.0 tumors/mouse for BALB-Min mice. The increase in tumor multiplicity was restricted to the small intestine. Only 1/60 (1.7%) BALB-RagMin mice were found to have colon tumors, and no cecal tumors were observed (Table 1). In contrast to those in B6-Min mice, the majority of tumors in BALB-RagMin mice were in the middle third of the small intestine (Table 2). BALB-RagMin small intestinal tumors did not differ significantly from those of BALB-Min mice at the gross morphological level. However, the median tumor sizes in BALB-RagMin mice were significantly smaller than those in BALB-Min mice in the middle and distal regions of the small intestine (Table 3). The median tumor sizes were also smaller in the BALB-RagMin mice's proximal small intestine regions, but this difference was not significant (Table 3).
Small intestine tumors of BALB-Min and BALB-RagMin were histologically similar by routine hematoxylin and eosin staining (data not shown). Loss of APC function results in the cytoplasmic accumulation and nuclear localization of β-catenin in intestinal tumors (55). To gain insight as to the underlying cause of the increase in small intestinal tumors, we stained BALB-Min and BALB-RagMin small intestinal tumors for the expression of β-catenin by using IHC. Similar β-catenin immunostaining patterns were found (Fig. 1C and D).
H. hepaticus induces moderate hepatitis in BALB-RagMin mice.
H. hepaticus infection results in severe typhlocolitis and development of cecal and colon adenocarcinomas in 129/SvEv-Rag2−/− mice (13). We tested the hypothesis that H. hepaticus infection would result in an increase in tumor multiplicity, a change in tumor regionality, or a change in tumor phenotype in BALB-RagMin mice. Fifty-eight BALB-RagMin mice were inoculated with H. hepaticus. Data from 24 H. hepaticus-infected mice were not evaluated, because the mice were euthanized prior to 12 wpi or died unexpectedly due to opportunistic infections (e.g., abscesses, conjunctivitis, or chronic loss of body condition). Because BALB-RagMin mice are immunodeficient and are prone to opportunistic infections (38), these deaths were not considered unusual. Indeed, similar deaths occurred among the uninoculated BALB-RagMin control mice. Of the remaining 34 BALB-RagMin mice, 13 (6 males and 7 females) were euthanized at 12 to 13 wpi and the remaining 21 (5 males and 16 females) were euthanized at 15 to 24 wpi to determine if length of infection affected tumorigenesis. All 34 mice were persistently infected with H. hepaticus, as determined by PCR on fecal samples obtained at necropsy (data not shown).
The livers from six H. hepaticus-infected BALB-RagMin mice (three males and three females) that were infected for 24 weeks and six uninfected BALB-RagMin mice (four males and two females) of the same age were processed for histology. H. hepaticus-infected BALB-RagMin mice had significantly higher median liver disease indices (9.5/16.0; range, 9.0 to 10.5) than uninfected BALB-RagMin mice (0.8/16.0; range, 0 to 2.5) (P < 0.0001; unpaired t test with Welch correction) (Fig. 1E and F). Hepatitis was arbitrarily defined as a liver disease index of >4.0. Based on this definition, all of the H. hepaticus-infected BALB-RagMin mice, and none of the uninfected mice, had hepatitis. Although the sample size is small, there was no suggestion of sexual dimorphism in liver lesion indices; the median and range of liver disease indices of H. hepaticus-infected males (median, 9.5/16.0; range, 9.0 to 10.0) were similar to those of H. hepaticus-infected females (median, 9.5/16.0; range, 9.0 to 10.5). Hepatic steatosis, dysplasia, and neoplasia were not observed.
H. hepaticus induces mild to moderate inflammation and hyperplasia of the cecum and colon in BALB-RagMin mice.
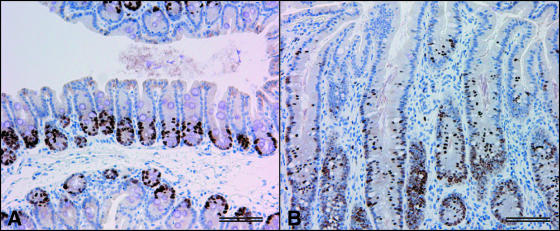
Ceca and colons of H. hepaticus-infected BALB-RagMin mice (4 to 7 months of age; 12 to 24 wpi; 2 males and 11 females) and 11 uninfected BALB-RagMin mice (4 to 8 months of age; 4 males and 7 females) were examined histologically. Relative to ceca from uninfected mice, the ceca of H. hepaticus-infected BALB-RagMin mice showed multifocal moderate inflammation with mononuclear cell infiltrates in the mucosa and submucosa. In uninfected mice, mitotic cells were usually found in the lower third of the cecal crypt, as demonstrated by immunostaining for the cell proliferation antigen Ki67 (Fig. 2A). In contrast, H. hepaticus-infected BALB-RagMin mice had Ki67-reactive cells in the upper half of the cecal crypt, an immunostaining pattern indicative of hyperplasia of the mucosal epithelium (Fig. 2B). Colon sections showed mild inflammation and hyperplasia that were multifocal in distribution (data not shown).
FIG. 2.
(A) Photomicrograph of a normal cecum from an uninfected BALB-RagMin mouse. Ki67-immunoreactive cells are localized to the lower third of the crypt. The cecal disease index was 0.5/8.0. (B) Photomicrograph of hyperplastic cecal tissue from an H. hepaticus-infected BALB-RagMin mouse at 24 wpi. Ki67-immunoreactive cells are present in the upper half of the crypt. The cecal disease index was 4.0/8.0. Scale bar = 100 μm.
To quantify the severity of the cecal and colon lesions and to determine if the lesions increased in severity over time, cecal and colon disease indices (maximum index = 8.0) were determined for four mice at 12 to 13 wpi and nine mice at 19 to 24 wpi. Median cecal and colon disease indices did not differ significantly at the two time points (P = 0.1398 and 0.1206 for cecal and colon indices, respectively; Mann-Whitney test), so the data for the two time points were pooled. H. hepaticus-infected BALB-RagMin mice had a small but significant increase in disease indices for the cecum (3.5/8.0; range, 3.0 to 5.5) and colon (2.0/8.0; range, 0 to 3.0) relative to uninfected BALB-RagMin mice (cecal disease index, 0.5/8.0 [range, 0 to 2.0] [P < 0.0001; Mann-Whitney test]; colon disease index, 0.5/8.0 [range, 0 to 3.5] [P = 0.027; Mann-Whitney test]).
H. hepaticus promotes colon tumorigenesis in BALB-RagMin mice.
Tumor multiplicity and regionality were determined for H. hepaticus-infected BALB-RagMin mice. Small intestine tumor multiplicity did not differ between mice at 12 to 13 wpi and mice at 15 to 24 wpi (P = 0.1276; Mann-Whitney test), so the data from both time points were pooled. Compared to what was found for uninfected BALB-RagMin mice, H. hepaticus infection did not affect either small intestine tumor multiplicity (Table 1) or regionality (Table 2). Although cecal tumors were found in 2/34 (5.9%) mice, cecal tumor incidence did not differ significantly from that in uninfected BALB-RagMin mice (P = 0.128; Fisher's exact test).
In contrast, H. hepaticus infection resulted in a significant increase in colon tumor incidence relative to that in uninfected BALB-RagMin mice (P = 0.001; Fisher's exact test). Colon tumors were found in 8/34 (23.5%) H. hepaticus-infected BALB-RagMin mice (Table 1) versus 1/60 (1.7%) uninfected BALB-RagMin mice. Hematoxylin and eosin staining and β-catenin IHC did not reveal any difference in staining patterns between the rare colon tumor identified in the uninfected BALB-RagMin mouse and the colon tumors from H. hepaticus-infected BALB-RagMin mice (data not shown).
The colon tumors were identified in one mouse at 19 wpi and seven mice at 23 to 24 wpi. These mice were 6 to 7 months of age. It was possible that colon tumor incidence simply increased with age regardless of H. hepaticus infection status. However, of 21 uninfected BALB-RagMin mice at 7 to 9 months of age, only 1 had a colon tumor. The difference in colon tumor incidence between H. hepaticus-infected BALB-RagMin mice ≥7 months of age (n = 17) and uninfected BALB-RagMin mice 7 to 9 months of age (n = 21) was statistically significant (P = 0.013; Fisher's exact test).
DISCUSSION
BALB/c mice were reported to have a resistant Mom1R locus (19). The low tumor multiplicities in both the small and large intestines of BALB-Min mice are consistent with the presence of a Mom1R locus. Genetic and transgenic studies suggest that at least one component of the Mom1 locus is the secretory phospholipase A2 group IIA gene (Pla2g2a) (7, 8, 36). All Mom1S strains tested to date have a single base pair insertion in Pla2g2a resulting in the absence of detectable Pla2g2a message and sPLA2-IIA activity, whereas all Mom1R strains, including BALB/c, have a wild-type Pla2g2a allele and high levels of sPLA2-IIA activity (19, 27, 36, 37). How the sPLA2-IIA protein confers tumor resistance remains to be determined.
Other congenic Min mouse strains have been reported. The AKR-Min congenic strain has a 25% tumor incidence, a very low small intestine tumor multiplicity (0.3 tumors/mouse), and a 0% prevalence of colon tumors. In addition, the AKR strain has a tendency to develop lymphomas, which not only complicates the interpretation of Min tumorigenesis studies but also contributes to a short life span (58). In contrast, the BALB-Min strain has a 100% tumor incidence, a median small intestine tumor multiplicity of 24.0 tumors/mouse, and a 5.9% prevalence of colon and cecal tumors. Although tumor multiplicity is considerably lower than that in the B6-Min strain, median tumor size was not significantly smaller, except in the distal region of the small intestine. More importantly, the BALB-Min strain has a considerably longer life span, with some mice living longer than 1 year. The availability of other Min congenic strains will allow the study of the effects of genetic background on the Min phenotype as shown here.
Although all cells of the B6-Min mouse are heterozygous for the mutant ApcMin allele, inactivation of the wild-type allele and the subsequent loss of APC function are required for tumor development (34, 35). It has been suggested that tumor regionality in the intestine reflects how APC function is lost (23). Three types of small intestine tumor regionality in Min mouse strains have been described. The first type, illustrated by B6-Min, is characterized by 75% of tumors developing in the distal half of the small intestine (23). In B6-Min mice, loss of APC function is associated with loss of heterozygosity by homologous somatic recombination (22, 34, 35). The second type is exemplified by AKR-Min mice. AKR-Min mice have a peak tumor distribution that is located even more distally, with 75% of tumors occurring in the last 20% of the small intestine (23). Loss of APC function in AKR-Min occurs predominantly through epigenetic silencing of the wild-type Apc allele, and only a small percentage of tumors show loss of heterozygosity (58). The third type, exemplified by the strain B6 Apc1628N, has tumors distributed evenly along the length of the small intestine. This tumor distribution pattern is associated with mutation of the wild-type allele (23). The majority of tumors in BALB-RagMin mice were found in the middle third of the small intestine (Table 2). Further studies will be needed to determine if this novel tumor distribution pattern represents a distinct mechanism for loss of APC function.
Tumor multiplicity in immunodeficient B6-Min Prkdcscid and B6-Min Lystbg−J mice did not differ significantly from that in immunocompetent B6-Min Prkdc+/+ and B6-Min Lyst+/+ mice (10, 11), leading to the conclusion that T and B cells of the adaptive immune system and NK cells of the innate immune system have minimal or no effect on ApcMin tumorigenesis. Our data show that immunodeficient BALB-RagMin mice have a significant increase in small, but not large, intestine tumors relative to immunocompetent BALB-Min mice. Similar results were recently reported for B6-Rag2−/− ApcMin/+ (B6-RagMin) mice (49), suggesting that the phenomenon is not specific to the BALB/c genetic background. These data indicate that in the BALB-Min small intestine the adaptive immune system plays a role in either the elimination (immunosurveillance) or the equilibrium (inhibition of tumor progression) phase of cancer immunoediting (12). Indeed, adoptive transfer of T-regulatory cells can induce tumor regression in B6-Min mice (14, 50). The absence of an increase in tumor multiplicity in the BALB-RagMin large intestine suggests that the roles of the adaptive immune system in tumorigenesis are different in the small and large intestines.
Microbe-induced inflammation has been linked to the promotion of cancer (26). H. hepaticus has been reported to promote tumorigenesis in B6-Min and B6-RagMin mice by an innate immune system-associated, tumor necrosis factor alpha-dependent process (49). In BALB-RagMin mice, H. hepaticus infection resulted in moderate hepatitis, multifocal moderate typhlitis, and multifocal mild colitis. Interestingly, a significant increase in tumor multiplicity was observed only in the colon, where inflammation was relatively mild. The data suggest that H. hepaticus promotion of tumorigenesis differs by organ and does not necessarily correlate with severity of inflammation. One explanation is that promotion of tumors in the liver and cecum may have a latency period greater than 24 wpi. Alternatively, H. hepaticus infection may promote colon tumors indirectly by modulating a carcinogenic factor in the colon. For example, human (3) and rodent (15, 46, 51) data suggest that secondary bile acids (deoxycholic acid and lithocholic acid) promote colon tumors. H. hepaticus may increase levels of secondary bile acids in the colon by increasing production of primary bile salts by the liver, by adversely affecting bile acid absorption from the distal ileum, and/or by modifying the large intestine bacterial microbiota in a manner that affects the activation and/or availability of secondary bile acids.
Our data show that the enterohepatic bacterial pathogen H. hepaticus promotes colon, but not small intestine, tumors in BALB-RagMin mice. These results corroborate data showing that H. hepaticus promotes azoxymethane-initiated colon tumors in the BALB-IL-10 knockout mouse (45). It has been reported that H. hepaticus can also promote mammary adenocarcinomas and small intestine tumors in B6-Min and B6-RagMin mice (49). Although we have identified mammary tumors in our BALB-RagMin mouse colony (data not shown), none of the H. hepaticus-infected BALB-RagMin mice developed a palpable mammary tumor. We also did not see any significant difference in small intestine tumor multiplicity or regionality (Tables 1 and 2). In addition, median tumor size did not differ significantly between uninfected and H. hepaticus-infected BALB-RagMin mice in either the proximal (2.1 mm versus 2.2 mm), the middle (1.5 mm versus 1.2 mm), or the distal (1.0 mm versus 1.0 mm) region of the small intestine. The reason for this discrepancy is unclear but may be due to background strain differences. Given that H. hepaticus is a common pathogen in many research mouse colonies (60), it is important to be aware that its presence may confound research using chemical carcinogenesis (azoxymethane) and genetic (ApcMin) mouse models for CRC.
Acknowledgments
We thank S. E. Erdman and V. P. Rao for their thoughtful comments on the manuscript and K. Schlieper, K. A. Knox, E. Groff, K. Cormier, K. Rydstrom, and C. Boussahamain for their technical assistance.
This work was supported by Public Health Service grants P30 ES002109 (J.G.F. and D.B.S.), T32 RR07036 and R01 CA67529 (J.G.F.), R01 DK52413 (D.B.S.), and P01 CA26731 (D.B.S. and J.G.F.).
Editor: B. A. McCormick
Footnotes
Published ahead of print on 14 April 2008.
REFERENCES
- 1.American Cancer Society. 2006. Cancer facts and figures 2006. American Cancer Society, Atlanta, GA.
- 2.Bassing, C. H., W. Swat, and F. W. Alt. 2002. The mechanism and regulation of chromosomal V(D)J. recombination. Cell 109(Suppl)S45-S55. [DOI] [PubMed] [Google Scholar]
- 3.Bayerdorffer, E., G. A. Mannes, T. Ochsenkuhn, P. Dirschedl, B. Wiebecke, and G. Paumgartner. 1995. Unconjugated secondary bile acids in the serum of patients with colorectal adenomas. Gut 36268-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boivin, G. P., K. Washington, K. Yang, J. M. Ward, T. P. Pretlow, R. Russell, D. G. Besselsen, V. L. Godfrey, T. Doetschman, W. F. Dove, H. C. Pitot, R. B. Halberg, S. H. Itzkowitz, J. Groden, and R. J. Coffey. 2003. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology 124762-777. [DOI] [PubMed] [Google Scholar]
- 5.Bourgade, F., X. Montagutelli, C. Bigbee, A. Weiss, L. Rigottier-Gois, C. J. Conti, and F. Benavides. 2004. Simple duplex fecal PCR assay that allows identification of false-negative results in Helicobacter sp.-infected mice. Comp. Med. 54528-532. [PubMed] [Google Scholar]
- 6.Burich, A., R. Hershberg, K. Waggie, W. Zeng, T. Brabb, G. Westrich, J. L. Viney, and L. Maggio-Price. 2001. Helicobacter-induced inflammatory bowel disease in IL-10- and T cell-deficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 281G764-G778. [DOI] [PubMed] [Google Scholar]
- 7.Cormier, R. T., A. Bilger, A. J. Lillich, R. B. Halberg, K. H. Hong, K. A. Gould, N. Borenstein, E. S. Lander, and W. F. Dove. 2000. The Mom1AKR intestinal tumor resistance region consists of Pla2g2a and a locus distal to D4Mit64. Oncogene 193182-3192. [DOI] [PubMed] [Google Scholar]
- 8.Cormier, R. T., K. H. Hong, R. B. Halberg, T. L. Hawkins, P. Richardson, R. Mulherkar, W. F. Dove, and E. S. Lander. 1997. Secretory phospholipase Pla2g2a confers resistance to intestinal tumorigenesis. Nat. Genet. 1788-91. [DOI] [PubMed] [Google Scholar]
- 9.Dietrich, W. F., E. S. Lander, J. S. Smith, A. R. Moser, K. A. Gould, C. Luongo, N. Borenstein, and W. Dove. 1993. Genetic identification of Mom-1, a major modifier locus affecting Min-induced intestinal neoplasia in the mouse. Cell 75631-639. [DOI] [PubMed] [Google Scholar]
- 10.Dove, W. F., L. Clipson, K. A. Gould, C. Luongo, D. J. Marshall, A. R. Moser, M. A. Newton, and R. F. Jacoby. 1997. Intestinal neoplasia in the ApcMin mouse: independence from the microbial and natural killer (beige locus) status. Cancer Res. 57812-814. [PubMed] [Google Scholar]
- 11.Dudley, M. E., J. P. Sundberg, and D. C. Roopenian. 1996. Frequency and histological appearance of adenomas in multiple intestinal neoplasia mice are unaffected by severe combined immunodeficiency (scid) mutation. Int. J. Cancer 65249-253. [DOI] [PubMed] [Google Scholar]
- 12.Dunn, G. P., L. J. Old, and R. D. Schreiber. 2004. The three Es of cancer immunoediting. Annu. Rev. Immunol. 22329-360. [DOI] [PubMed] [Google Scholar]
- 13.Erdman, S. E., T. Poutahidis, M. Tomczak, A. B. Rogers, K. Cormier, B. Plank, B. H. Horwitz, and J. G. Fox. 2003. CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am. J. Pathol. 162691-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erdman, S. E., J. J. Sohn, V. P. Rao, P. R. Nambiar, Z. Ge, J. G. Fox, and D. B. Schauer. 2005. CD4+CD25+ regulatory lymphocytes induce regression of intestinal tumors in ApcMin/+ mice. Cancer Res. 653998-4004. [DOI] [PubMed] [Google Scholar]
- 15.Flynn, C., D. C. Montrose, D. L. Swank, M. Nakanishi, J. N. Ilsley, and D. W. Rosenberg. 2007. Deoxycholic acid promotes the growth of colonic aberrant crypt foci. Mol. Carcinog. 4660-70. [DOI] [PubMed] [Google Scholar]
- 16.Fox, J. G., F. E. Dewhirst, Z. Shen, Y. Feng, N. S. Taylor, B. J. Paster, R. L. Ericson, C. N. Lau, P. Correa, J. C. Araya, and I. Roa. 1998. Hepatic Helicobacter species identified in bile and gallbladder tissue from Chileans with chronic cholecystitis. Gastroenterology 114755-763. [DOI] [PubMed] [Google Scholar]
- 17.Fox, J. G., F. E. Dewhirst, J. G. Tully, B. J. Paster, L. Yan, N. S. Taylor, M. J. Collins, Jr., P. L. Gorelick, and J. M. Ward. 1994. Helicobacter hepaticus sp. nov., a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J. Clin. Microbiol. 321238-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox, J. G., X. Li, L. Yan, R. J. Cahill, R. Hurley, R. Lewis, and J. C. Murphy. 1996. Chronic proliferative hepatitis in A/JCr mice associated with persistent Helicobacter hepaticus infection: a model of helicobacter-induced carcinogenesis. Infect. Immun. 641548-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gould, K. A., C. Luongo, A. R. Moser, M. K. McNeley, N. Borenstein, A. Shedlovsky, W. F. Dove, K. Hong, W. F. Dietrich, and E. S. Lander. 1996. Genetic evaluation of candidate genes for the Mom1 modifier of intestinal neoplasia in mice. Genetics 1441777-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gounari, F., R. Chang, J. Cowan, Z. Guo, M. Dose, E. Gounaris, and K. Khazaie. 2005. Loss of adenomatous polyposis coli gene function disrupts thymic development. Nat. Immunol. 6800-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groden, J., A. Thliveris, W. Samowitz, M. Carlson, L. Gelbert, H. Albertsen, G. Joslyn, J. Stevens, L. Spirio, M. Robertson, L. Sargeant, K. Krapcho, E. Wolff, R. Burt, J. P. Hughes, J. Warrington, J. McPherson, J. Wasmuth, D. Le Paslier, H. Abderrahim, D. Cohen, M. Leppert, and R. White. 1991. Identification and characterization of the familial adenomatous polyposis coli gene. Cell 66589-600. [DOI] [PubMed] [Google Scholar]
- 22.Haigis, K. M., J. G. Caya, M. Reichelderfer, and W. F. Dove. 2002. Intestinal adenomas can develop with a stable karyotype and stable microsatellites. Proc. Natl. Acad. Sci. USA 998927-8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haigis, K. M., P. D. Hoff, A. White, A. R. Shoemaker, R. B. Halberg, and W. F. Dove. 2004. Tumor regionality in the mouse intestine reflects the mechanism of loss of Apc function. Proc. Natl. Acad. Sci. USA 1019769-9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inomata, M., A. Ochiai, S. Akimoto, S. Kitano, and S. Hirohashi. 1996. Alteration of β-catenin expression in colonic epithelial cells of familial adenomatous polyposis patients. Cancer Res. 562213-2217. [PubMed] [Google Scholar]
- 25.Ishikawa, T. O., Y. Tamai, Q. Li, M. Oshima, and M. M. Taketo. 2003. Requirement for tumor suppressor Apc in the morphogenesis of anterior and ventral mouse embryo. Dev. Biol. 253230-246. [DOI] [PubMed] [Google Scholar]
- 26.Karin, M., T. Lawrence, and V. Nizet. 2006. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell 124823-835. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy, B. P., P. Payette, J. Mudgett, P. Vadas, W. Pruzanski, M. Kwan, C. Tang, D. E. Rancourt, and W. A. Cromlish. 1995. A natural disruption of the secretory group II phospholipase A2 gene in inbred mouse strains. J. Biol. Chem. 27022378-22385. [DOI] [PubMed] [Google Scholar]
- 28.Kinzler, K. W., M. C. Nilbert, L. K. Su, B. Vogelstein, T. M. Bryan, D. B. Levy, K. J. Smith, A. C. Preisinger, P. Hedge, D. McKechnie, R. Finniear, A. Markham, J. Groffen, M. S. Boguski, S. F. Altschul, A. Horii, H. Ando, Y. Miyoshi, Y. Miki, I. Nishisho, and Y. Nakamura. 1991. Identification of FAP locus genes from chromosome 5q21. Science 253661-665. [DOI] [PubMed] [Google Scholar]
- 29.Kinzler, K. W., and B. Vogelstein. 1996. Lessons from hereditary colorectal cancer. Cell 87159-170. [DOI] [PubMed] [Google Scholar]
- 30.Koratkar, R., K. A. Silverman, E. Pequignot, W. W. Hauck, A. M. Buchberg, and L. D. Siracusa. 2004. Analysis of reciprocal congenic lines reveals the C3H/HeJ genome to be highly resistant to ApcMin intestinal tumorigenesis. Genomics 84844-852. [DOI] [PubMed] [Google Scholar]
- 31.Korinek, V., N. Barker, P. J. Morin, D. van Wichen, R. de Weger, K. W. Kinzler, B. Vogelstein, and H. Clevers. 1997. Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science 2751784-1787. [DOI] [PubMed] [Google Scholar]
- 32.Kullberg, M. C., J. M. Ward, P. L. Gorelick, P. Caspar, S. Hieny, A. Cheever, D. Jankovic, and A. Sher. 1998. Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism. Infect. Immun. 665157-5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuraguchi, M., X. P. Wang, R. T. Bronson, R. Rothenberg, N. Y. Ohene-Baah, J. J. Lund, M. Kucherlapati, R. L. Maas, and R. Kucherlapati. 2006. Adenomatous polyposis coli (APC) is required for normal development of skin and thymus. PLoS Genet. 21362-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levy, D. B., K. J. Smith, Y. Beazer-Barclay, S. R. Hamilton, B. Vogelstein, and K. W. Kinzler. 1994. Inactivation of both APC alleles in human and mouse tumors. Cancer Res. 545953-5958. [PubMed] [Google Scholar]
- 35.Luongo, C., A. R. Moser, S. Gledhill, and W. F. Dove. 1994. Loss of Apc+ in intestinal adenomas from Min mice. Cancer Res. 545947-5952. [PubMed] [Google Scholar]
- 36.MacPhee, M., K. P. Chepenik, R. A. Liddell, K. K. Nelson, L. D. Siracusa, and A. M. Buchberg. 1995. The secretory phospholipase A2 gene is a candidate for the Mom1 locus, a major modifier of ApcMin-induced intestinal neoplasia. Cell 81957-966. [DOI] [PubMed] [Google Scholar]
- 37.Markova, M., R. A. Koratkar, K. A. Silverman, V. E. Sollars, M. MacPhee-Pellini, R. Walters, J. P. Palazzo, A. M. Buchberg, L. D. Siracusa, and S. A. Farber. 2005. Diversity in secreted PLA2-IIA activity among inbred mouse strains that are resistant or susceptible to ApcMin/+ tumorigenesis. Oncogene 246450-6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mombaerts, P. 1995. Lymphocyte development and function in T-cell receptor and RAG-1 mutant mice. Int. Rev. Immunol. 1343-63. [DOI] [PubMed] [Google Scholar]
- 39.Mombaerts, P., J. Iacomini, R. S. Johnson, K. Herrup, S. Tonegawa, and V. E. Papaioannou. 1992. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68869-877. [DOI] [PubMed] [Google Scholar]
- 40.Morin, P. J., A. B. Sparks, V. Korinek, N. Barker, H. Clevers, B. Vogelstein, and K. W. Kinzler. 1997. Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science 2751787-1790. [DOI] [PubMed] [Google Scholar]
- 41.Moser, A. R., W. F. Dove, K. A. Roth, and J. I. Gordon. 1992. The Min (multiple intestinal neoplasia) mutation: its effect on gut epithelial cell differentiation and interaction with a modifier system. J. Cell Biol. 1161517-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moser, A. R., C. Luongo, K. A. Gould, M. K. McNeley, A. R. Shoemaker, and W. F. Dove. 1995. ApcMin: a mouse model for intestinal and mammary tumorigenesis. Eur. J. Cancer. 31A1061-1064. [DOI] [PubMed] [Google Scholar]
- 43.Moser, A. R., H. C. Pitot, and W. F. Dove. 1990. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science 247322-324. [DOI] [PubMed] [Google Scholar]
- 44.Moser, A. R., A. R. Shoemaker, C. S. Connelly, L. Clipson, K. A. Gould, C. Luongo, W. F. Dove, P. H. Siggers, and R. L. Gardner. 1995. Homozygosity for the Min allele of Apc results in disruption of mouse development prior to gastrulation. Dev. Dyn. 203422-433. [DOI] [PubMed] [Google Scholar]
- 45.Nagamine, C. M., A. B. Rogers, J. G. Fox, and D. B. Schauer. 2008. Helicobacter hepaticus promotes azoxymethane-initiated colon tumorigenesis in BALB/c-IL10-deficient mice. Int. J. Cancer. 122832-838. [DOI] [PubMed] [Google Scholar]
- 46.Narisawa, T., N. E. Magadia, J. H. Weisburger, and E. L. Wynder. 1974. Promoting effect of bile acids on colon carcinogenesis after intrarectal instillation of N-methyl-N′-nitro-N-nitrosoguanidine in rats. J. Natl. Cancer Inst. 531093-1097. [DOI] [PubMed] [Google Scholar]
- 47.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 48.Powell, S. M., N. Zilz, Y. Beazer-Barclay, T. M. Bryan, S. R. Hamilton, S. N. Thibodeau, B. Vogelstein, and K. W. Kinzler. 1992. APC mutations occur early during colorectal tumorigenesis. Nature 359235-237. [DOI] [PubMed] [Google Scholar]
- 49.Rao, V. P., T. Poutahidis, Z. Ge, P. R. Nambiar, C. Boussahmain, Y. Y. Wang, B. H. Horwitz, J. G. Fox, and S. E. Erdman. 2006. Innate immune inflammatory response against enteric bacteria Helicobacter hepaticus induces mammary adenocarcinoma in mice. Cancer Res. 667395-7400. [DOI] [PubMed] [Google Scholar]
- 50.Rao, V. P., T. Poutahidis, Z. Ge, P. R. Nambiar, B. H. Horwitz, J. G. Fox, and S. E. Erdman. 2006. Proinflammatory CD4+ CD45RBhi lymphocytes promote mammary and intestinal carcinogenesis in ApcMin/+ mice. Cancer Res. 6657-61. [DOI] [PubMed] [Google Scholar]
- 51.Reddy, B. S., T. Narasawa, J. H. Weisburger, and E. L. Wynder. 1976. Promoting effect of sodium deoxycholate on colon adenocarcinomas in germfree rats. J. Natl. Cancer Inst. 56441-442. [DOI] [PubMed] [Google Scholar]
- 52.Roberts, R. B., L. Min, M. K. Washington, S. J. Olsen, S. H. Settle, R. J. Coffey, and D. W. Threadgill. 2002. Importance of epidermal growth factor receptor signaling in establishment of adenomas and maintenance of carcinomas during intestinal tumorigenesis. Proc. Natl. Acad. Sci. USA 991521-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sansom, O. J., V. S. Meniel, V. Muncan, T. J. Phesse, J. A. Wilkins, K. R. Reed, J. K. Vass, D. Athineos, H. Clevers, and A. R. Clarke. 2007. Myc deletion rescues Apc deficiency in the small intestine. Nature 446676-679. [DOI] [PubMed] [Google Scholar]
- 54.Scholzen, T., and J. Gerdes. 2000. The Ki-67 protein: from the known and the unknown. J. Cell. Physiol. 182311-322. [DOI] [PubMed] [Google Scholar]
- 55.Sheng, H., J. Shao, C. S. Williams, M. A. Pereira, M. M. Taketo, M. Oshima, A. B. Reynolds, M. K. Washington, R. N. DuBois, and R. D. Beauchamp. 1998. Nuclear translocation of β-catenin in hereditary and carcinogen-induced intestinal adenomas. Carcinogenesis 19543-549. [DOI] [PubMed] [Google Scholar]
- 56.Shinkai, Y., G. Rathbun, K. P. Lam, E. M. Oltz, V. Stewart, M. Mendelsohn, J. Charron, M. Datta, F. Young, A. M. Stall, and F. W. Alt. 1992. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J. rearrangement. Cell 68855-867. [DOI] [PubMed] [Google Scholar]
- 57.Shoemaker, A. R., A. R. Moser, and W. F. Dove. 1995. N-ethyl-N-nitrosourea treatment of multiple intestinal neoplasia (Min) mice: age-related effects on the formation of intestinal adenomas, cystic crypts, and epidermoid cysts. Cancer Res. 554479-4485. [PubMed] [Google Scholar]
- 58.Shoemaker, A. R., A. R. Moser, C. A. Midgley, L. Clipson, M. A. Newton, and W. F. Dove. 1998. A resistant genetic background leading to incomplete penetrance of intestinal neoplasia and reduced loss of heterozygosity in ApcMin/+ mice. Proc. Natl. Acad. Sci. USA 9510826-10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Su, L. K., K. W. Kinzler, B. Vogelstein, A. C. Preisinger, A. R. Moser, C. Luongo, K. A. Gould, and W. F. Dove. 1992. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science 256668-670. [DOI] [PubMed] [Google Scholar]
- 60.Taylor, N. S., S. Xu, P. Nambiar, F. E. Dewhirst, and J. G. Fox. 2007. Enterohepatic Helicobacter species are prevalent in mice from commercial and academic institutions in Asia, Europe, and North America. J. Clin. Microbiol. 452166-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ward, J. M., J. G. Fox, M. R. Anver, D. C. Haines, C. V. George, M. J. Collins, Jr., P. L. Gorelick, K. Nagashima, M. A. Gonda, R. V. Gilden, J. G. Tully, R. J. Russell, R. E. Benveniste, B. J. Paster, F. E. Dewhirst, J. C. Donovan, L. M. Anderson, and J. M. Rice. 1994. Chronic active hepatitis and associated liver tumors in mice caused by a persistent bacterial infection with a novel Helicobacter species. J. Natl. Cancer Inst. 861222-1227. [DOI] [PubMed] [Google Scholar]
- 62.Wilson, C. L., K. J. Heppner, P. A. Labosky, B. L. Hogan, and L. M. Matrisian. 1997. Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc. Natl. Acad. Sci. USA 941402-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]