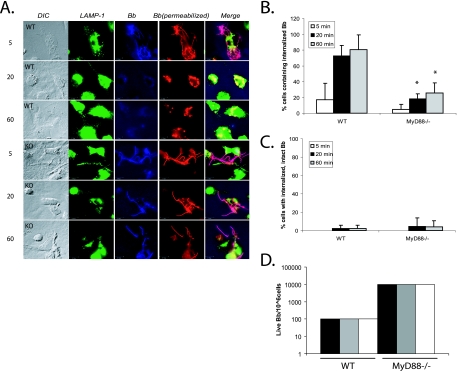
FIG. 1.
MyD88−/− BMDM show deficient uptake of B. burgdorferi. (A) BMDM from wild-type littermates and MyD88−/− mice were isolated. B. burgdorferi spirochetes were added to the BMDM at an MOI of 10. The cells were incubated with B. burgdorferi for 5, 20, or 60 min. Cells were fixed at each time point and then probed with various antibodies. Lanes: DIC, differential interference contrast; LAMP-1, anti-LAMP-1 (lysosomal marker) antibody (green); Bb, rabbit anti-B. burgdorferi antibody prior to permeabilization of the cells to identify extracellular organisms (blue); Bb (permeabilized), rabbit anti-B. burgdorferi antibody after permeabilization of the cells to identify both internalized and extracellular B. burgdorferi epitopes (red). Lane Merge shows a merged image of LAMP-1, Bb, and Bb (permeabilized). Experiments were repeated five times with cells from two different mice per experiment, and representative images are shown. Scale bar, 10 μM. (B) Cells containing internalized B. burgdorferi particles were counted and expressed as a percentage of the total number of cells examined. (C) Cells containing spirochetes that appeared intact and not degraded (i.e., that maintained their spirochetal architecture) were counted and expressed as a percentage of the total cells. Each experiment was repeated five times using cells from two different mice each time. The error bars represent standard deviations. *, P ≤ 0.05 compared with wild-type (WT) cells at matched postinfection time points. (D) For quantitative analysis of B. burgdorferi survival in the BMDM from wild-type littermates and MyD88−/− mice, B. burgdorferi spirochetes were incubated with macrophages as before. After 1 h of incubation, the cells were washed three times with cold PBS to eliminate unbound B. burgdorferi spirochetes. Cells (106) were resuspended in BSK liquid medium and serially diluted in 96-well plates. The wells were monitored for growth of B. burgdorferi. Experiments were performed in duplicate and repeated three times with cells from two different mice per experiment. Each bar represents one independent experiment.