Abstract
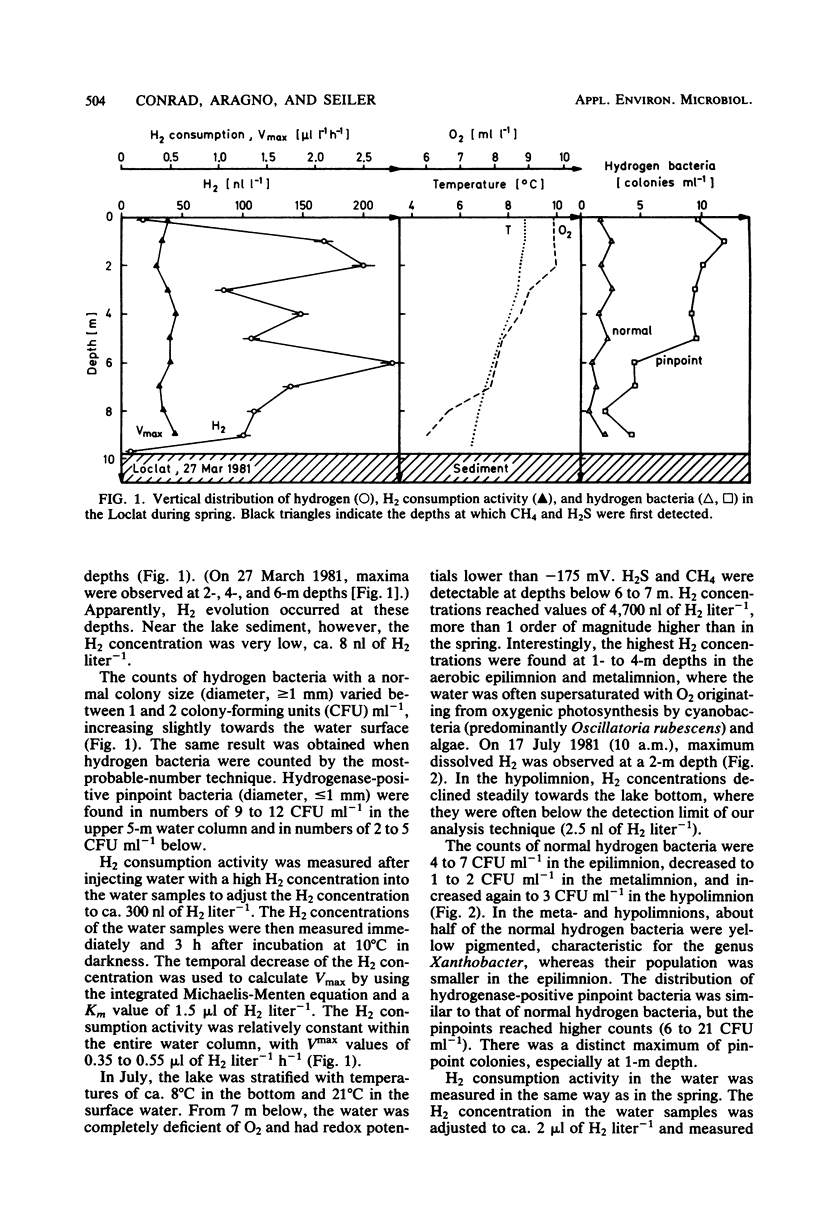
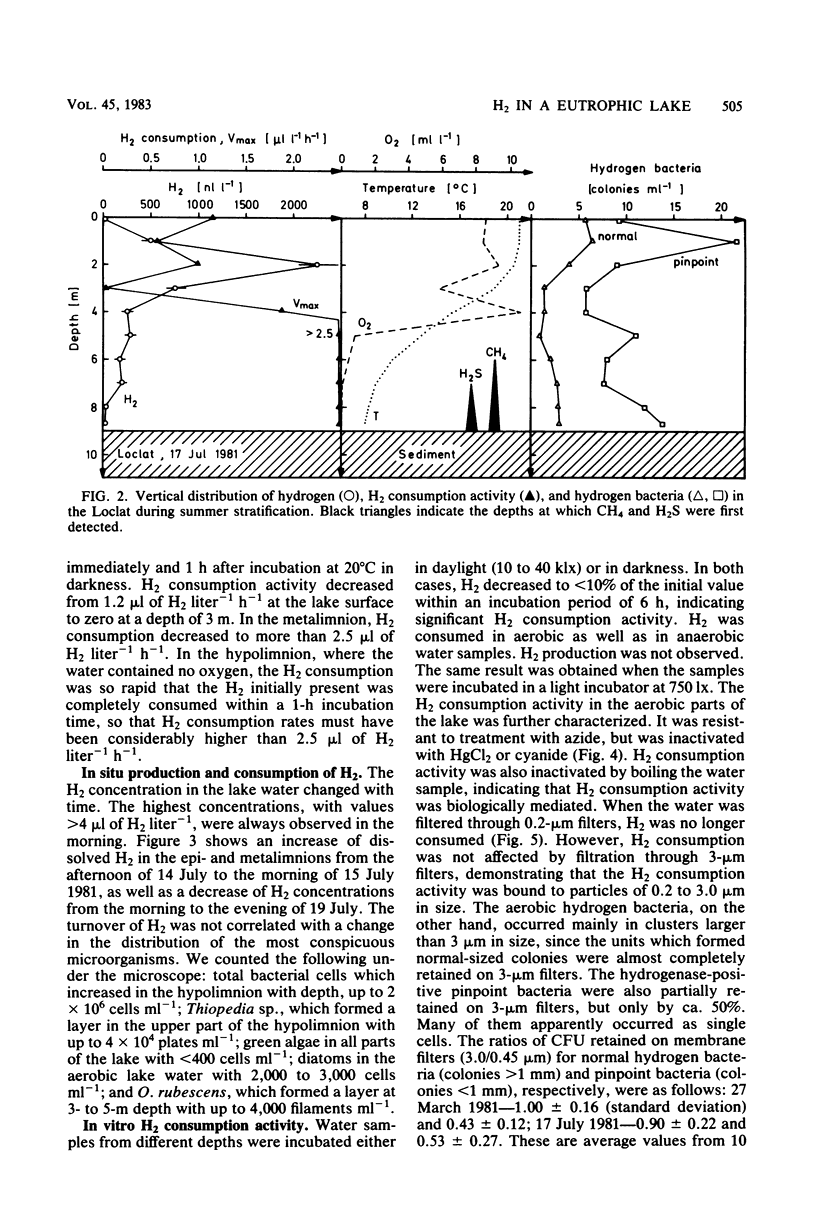
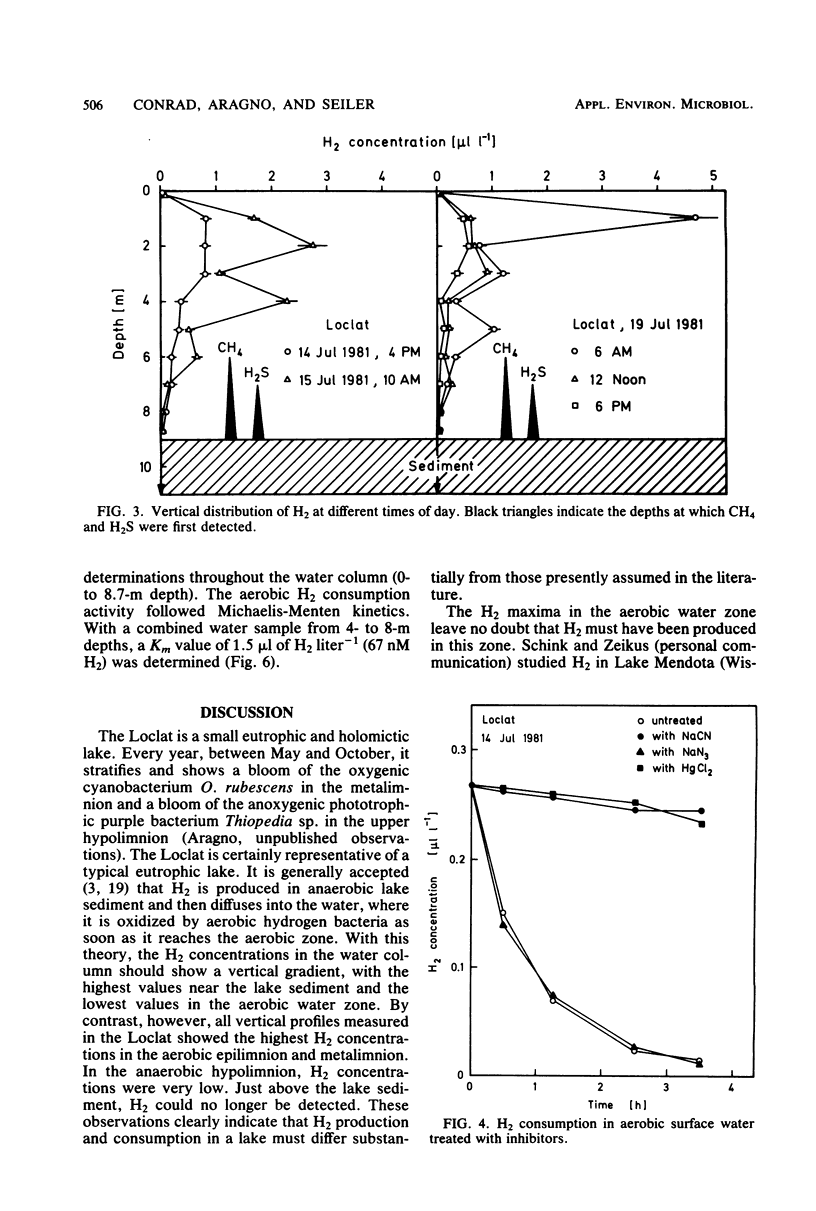
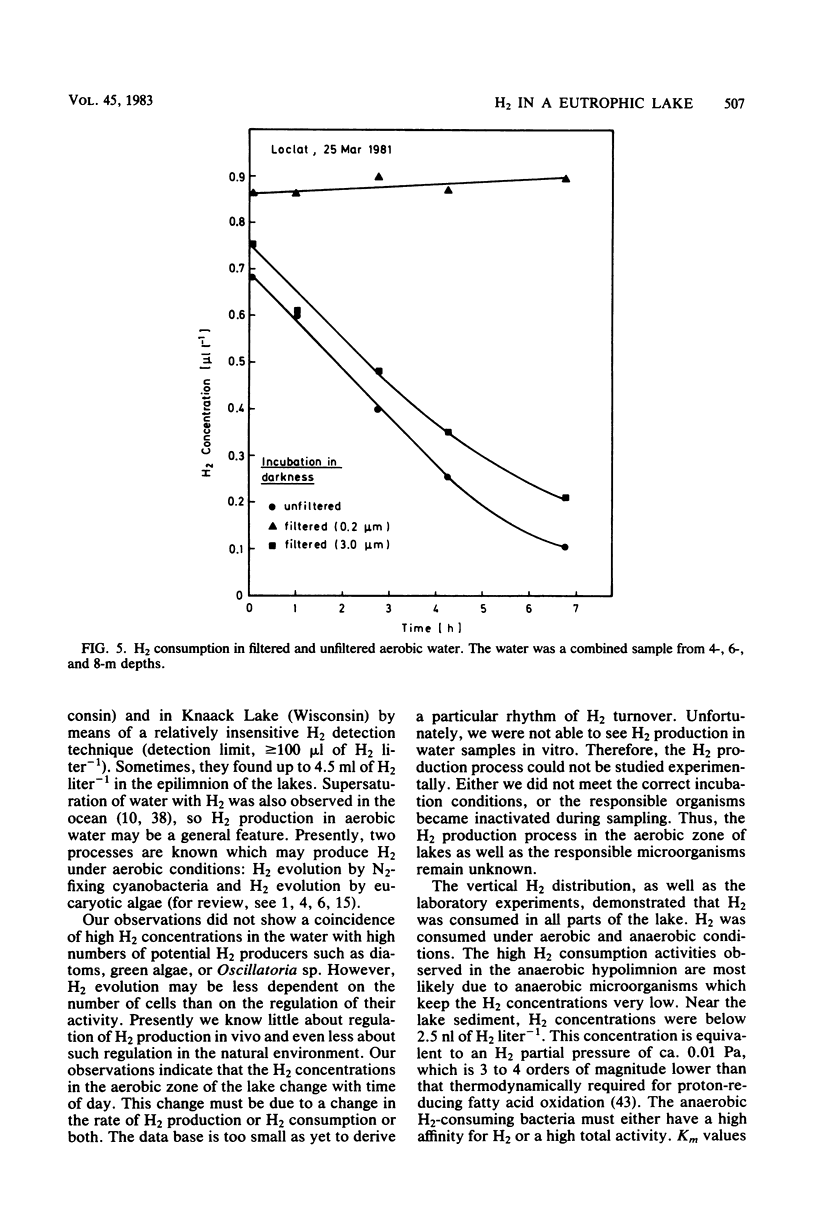
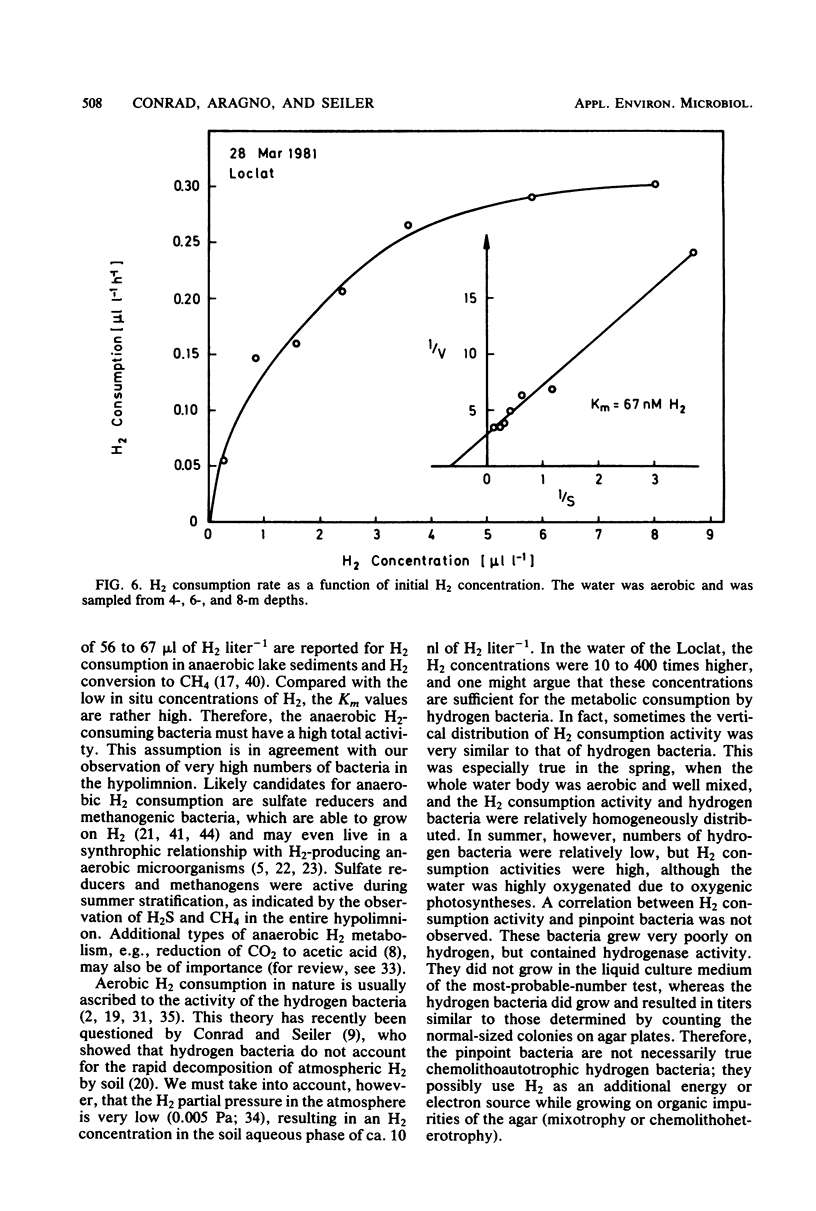
The vertical distribution of hydrogen was measured in the Loclat, a eutrophic and holomictic lake near Neuchâtel, Switzerland, before and during summer stratification. H2 concentrations decreased with depth in the anaerobic hypolimnion and were often below the detection limit (2.5 nl of H2 liter−1) in the water adjacent to the lake sediment. H2 was apparently not released from the lake sediment. The highest H2 concentrations (>4 μl of H2 liter−1) were observed in the aerobic water of the epilimnion and metalimnion. There, the H2 concentrations changed with time, indicating a turnover of H2. The H2 production processes could not be studied in the laboratory since incubation of water samples in light or darkness did not result in H2 production but rather always in H2 consumption. The possible role of cyanobacteria and algae for H2 production is discussed. Aerobic or anaerobic H2 consumption activities were observed at all depths of the water column, with highest activities in the hypolimnion. Aerobic H2 consumption activity was insensitive to azide inhibition, but sensitive to heat, mercuric chloride, or cyanide. It was restricted to a particle fraction of 0.2 to 3.0 μm in size, so that it must be due to single bacterial cells. Aerobic hydrogen bacteria, on the other hand, occurred in clusters of >3.0 μm. Therefore, the hydrogen bacteria could not have caused the H2 consumption in lake water. The aerobic H2 consumption activity followed Michaelis-Menten kinetics, with a Km of 67 nM H2. This is an exceptionally low value compared with Km values of hydrogenases in hydrogen bacteria and other species, but is similar to that for H2-decomposing abiontic soil hydrogenases.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. W., Mortenson L. E., Chen J. S. Hydrogenase. Biochim Biophys Acta. 1980 Dec;594(2-3):105–176. doi: 10.1016/0304-4173(80)90007-5. [DOI] [PubMed] [Google Scholar]
- Boone D. R., Bryant M. P. Propionate-Degrading Bacterium, Syntrophobacter wolinii sp. nov. gen. nov., from Methanogenic Ecosystems. Appl Environ Microbiol. 1980 Sep;40(3):626–632. doi: 10.1128/aem.40.3.626-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowien B., Schlegel H. G. Physiology and biochemistry of aerobic hydrogen-oxidizing bacteria. Annu Rev Microbiol. 1981;35:405–452. doi: 10.1146/annurev.mi.35.100181.002201. [DOI] [PubMed] [Google Scholar]
- Braun M., Schoberth S., Gottschalk G. Enumeration of bacteria forming acetate from H2 and CO2 in anaerobic habitats. Arch Microbiol. 1979 Mar 12;120(3):201–204. doi: 10.1007/BF00423066. [DOI] [PubMed] [Google Scholar]
- Kirchman D., Mitchell R. Contribution of particle-bound bacteria to total microheterotrophic activity in five ponds and two marshes. Appl Environ Microbiol. 1982 Jan;43(1):200–209. doi: 10.1128/aem.43.1.200-209.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah R. A., Ward D. M., Baresi L., Glass T. L. Biogenesis of methane. Annu Rev Microbiol. 1977;31:309–341. doi: 10.1146/annurev.mi.31.100177.001521. [DOI] [PubMed] [Google Scholar]
- McInerney M. J., Bryant M. P., Hespell R. B., Costerton J. W. Syntrophomonas wolfei gen. nov. sp. nov., an Anaerobic, Syntrophic, Fatty Acid-Oxidizing Bacterium. Appl Environ Microbiol. 1981 Apr;41(4):1029–1039. doi: 10.1128/aem.41.4.1029-1039.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfennig N. Photosynthetic bacteria. Annu Rev Microbiol. 1967;21:285–324. doi: 10.1146/annurev.mi.21.100167.001441. [DOI] [PubMed] [Google Scholar]
- Powell M. R., Doebbler G. F., Hamilton R. W., Jr Serum enzyme level changes in pigs following decompression trauma. Aerosp Med. 1974 May;45(5):519–524. [PubMed] [Google Scholar]
- SCHLEGEL H. G., KALTWASSER H., GOTTSCHALK G. [A submersion method for culture of hydrogen-oxidizing bacteria: growth physiological studies]. Arch Mikrobiol. 1961;38:209–222. [PubMed] [Google Scholar]
- Strayer R. F., Tiedje J. M. Kinetic parameters of the conversion of methane precursors to methane in a hypereutrophic lake sediment. Appl Environ Microbiol. 1978 Aug;36(2):330–340. doi: 10.1128/aem.36.2.330-340.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G. The biology of methanogenic bacteria. Bacteriol Rev. 1977 Jun;41(2):514–541. doi: 10.1128/br.41.2.514-541.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


