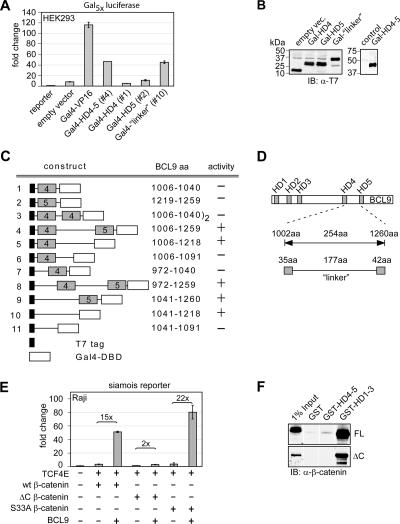
FIG. 5.
The BCL9 C terminus is sufficient to activate a synthetic Gal4 reporter and requires the C-terminal transactivation domain of β-catenin. (A) Part of the BCL9 C terminus fused to the Gal4 DBD suffices to activate a synthetic reporter consisting of multimerized Gal4 DBD-binding sites upstream of luciferase and thereby defines a transactivation domain. The indicated gene constructs (0.1 μg each) were transiently transfected into HEK293 cells. (B) Immunoblot analysis (IB) of T7 epitope-labeled fusion proteins confirms similar expression levels. Indicated constructs were transiently expressed in HEK293 cells, and whole-cell extract was analyzed. α-T7, anti-T7. (C) Delineation of the transactivation domain of BCL9 in fusions with the Gal4 DBD. Numbers indicate the amino acid sequences of BCL9. “Activity” indicates the ability of the respective construct to activate a synthetic Gal4 reporter in transfection experiments. (D) Schematic representation of the BCL9 transactivation domain. Homology domains (HD) 4 and 5 are marked according to their homology to BCL9-2. (E) BCL9-mediated transactivation depends on the presence of the C-terminal transactivation domain of β-catenin. To allow for similar protein accumulations of wild-type and mutant β-catenin (data not shown), 3 μg of the wild-type β-catenin gene construct or 0.3 μg of the S33A or ΔC β-catenin gene constructs was transfected, together with 0.5 μg of the BCL9 construct as indicated. (F) Recombinantly purified full-length (FL) and ΔC-β-catenin interact with GST-HD1-3 of BCL9 but not HD4-5 in a GST pulldown assay. β-Catenin was visualized by immunoblot analysis.