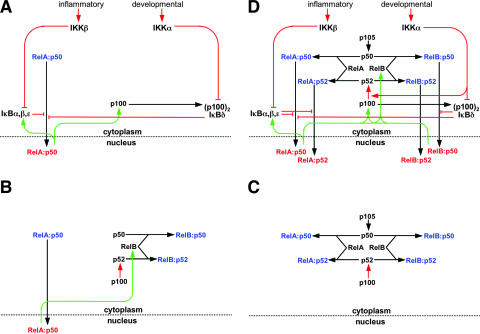
FIG. 7.
Wiring diagrams to depict functional relationships within the NF-κB signaling system. NF-κB monomers are shown in black, cytoplasmic dimers are in blue, and nuclear dimers are in red. Constitutive processes are shown in black, regulated processes are in red, and feedback processes are in green. (A) A wiring diagram of the regulation of the NF-κB/RelA-p50 dimer in response to inflammatory or developmental stimuli. A mathematical model of these functional connections has been constructed and shown to recapitulate signaling cross talk between these pathways in the control of RelA-p50 activity (2). (B) A wiring diagram to summarize our findings (Fig. 2 and 3) that steady-state RelB synthesis and the availability of latent RelB dimers are dependent on constitutive RelA-p50 activity. In Fig. 4, we also showed that constitutive RelA-p50 activity is controlled by constitutive IKKβ activity. (C) A wiring diagram to describe the molecular competition between p50 and p52 for their dimerization partners RelA and RelB. This competition determines the rate of p50 and p52 generation via processing from their p105 and p100 precursors. Thus, p105/p50 and p100/p52 processing is interdependent through competition for common interaction partners. (D) A wiring diagram of the NF-κB signaling system that accounts for the generation of four NF-κB dimers that are detected in response to LTβR signaling, namely, RelA-p50, RelA-p52, RelB-p50, and RelB-p52. This diagram is based on previously established connectivity (A) and incorporates the insights from the current study summarized in panels B and C. The NF-κB signaling system receives signals from both inflammatory and developmental cues through IKK2/IKKβ and IKK1/IKKα kinases, respectively, to activate distinct NF-κB dimers. For the sake of clarity, we have depicted only signal-responsive protein complexes, omitting dimers such as p50-p50, p105-p50, p105-RelA, p100-p52, or p100-RelB that do not change in abundance during LTβR induced signaling.