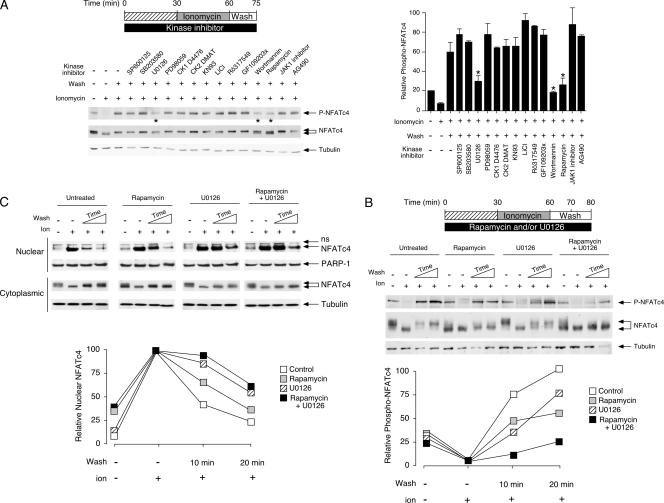
FIG. 4.
Identification of protein kinases targeting NFATc4 Ser168,170 upon rephosphorylation. (A) Rapamycin, wortmannin, or U0126 blocks rephosphorylation at Ser168,170 of NFATc4. MEFs were serum starved for 2 h before incubation with various protein kinase inhibitors as indicated. Cells were then stimulated with ionomycin for 30 min before the removal of ionomycin and subsequent wash (15 min). Phosphorylation at Ser168,170 of endogenous NFATc4 was determined by phospho-NFATc4 monoclonal antibody (P-NFATc4). The expression of NFATc4 and tubulin is also shown. The relative intensity of NFATc4 phosphorylation (P-NFATc4/NFATc4) was quantified by ImageQuant software and presented (mean ± SD [n = 3]). See the legend to Fig. 3 for the concentration of protein kinase inhibitors administered. (B and C) The effects of rapamycin and U0126 in NFATc4 rephosphorylation and nuclear export. MEFs were serum starved for 2 h before incubation with rapamycin (100 nM) and/or U0126 (5 μM), as indicated. Treated cells were stimulated with ionomycin for 30 min before the removal of ionomycin and subsequent wash (10 or 20 min). Phosphorylation at Ser168,170 of endogenous NFATc4 was determined by phospho-NFATc4 monoclonal antibody (P-NFATc4) (B). The electrophoretic mobility of NFATc4 was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (B). The levels of NFATc4 in nuclear and cytoplasmic fractions are also shown (C). The relative intensities of phospho-NFATc4 and nuclear NFATc4 (nucleocytoplasmic plus nuclear) were quantified by ImageQuant software and presented. The results are representative of three independent experiments. ns, nonspecific cross-reactivity.