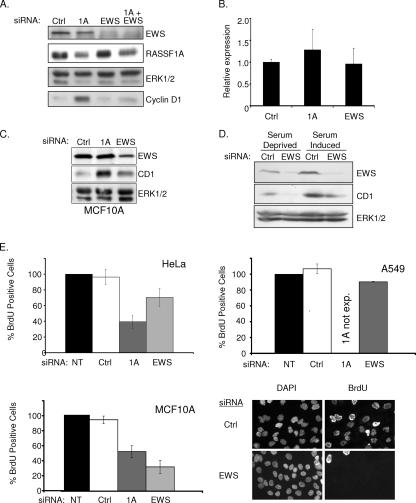
FIG. 2.
EWS regulates cyclin D1 expression. (A) HeLa cells were transfected with the indicated siRNAs in the presence of serum. Seventy-two hours later, lysates were resolved by SDS-PAGE and immunoblotted for indicated proteins. (B) Cyclin D1 mRNA concentrations from cells treated as described for panel A were evaluated by quantitative reverse transcription-PCR. Error bars represent the standard deviation from the mean of three biological replicates. (C) MCF10A cells were transfected with the indicated siRNAs. Seventy-two hours later, whole-cell lysates were immunoblotted to visualize the indicated proteins. ERK1/2 was included as a loading control (Ctrl). (D) HeLa cells were transfected as described for panel A, with the exception that cells were incubated in serum-free medium for 24 h prior to lysate collection as indicated. ERK1/2 was included as a loading control. (E) The indicated cell lines were transfected as described for panel A. At 48 h following transfection, BrdU was added to the medium for an additional 24 h. BrdU incorporation was detected with an anti-BrdU antibody, nuclei were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI), and the percentage of BrdU-positive cells was calculated by microscopic observation. At least 100 cells were analyzed for each condition in each experiment. Values were normalized to the BrdU incorporation frequency observed in untransfected (NT) cells, which was arbitrarily set to 100%. Error bars indicate the standard deviation from the mean of three biological replicates. Representative micrographs are shown for MCF10A cells at the bottom left. exp., expressed.