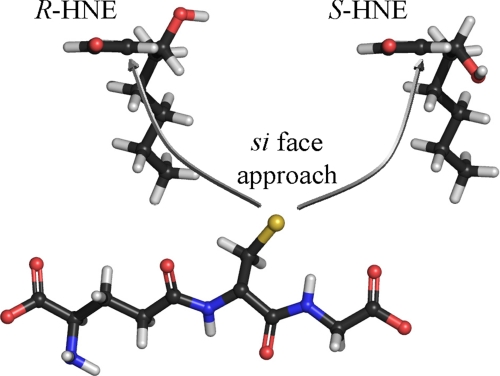
FIGURE 7.
Stereoselective GSH conjugation to HNE catalyzed by hGSTA4-4. Both enantiomers of HNE as viewed directly along the 2,3-double bond axis, showing the relative orientation of the GSH thiolate and the 2,3-double bond of HNE within the context of the hGSTA4-4 active site. To achieve the observed product stereoselectivity, the nucleophilic attack occurs from the si face of the double bond for both HNE enantiomers resulting in the S-configuration at the site of conjugation. This orientation places the hydroxyl group of each enantiomer on opposite sides of the active site. Thus, the hydroxyl group is either not exploited for binding, or else there are interactions that are energetically comparable, such as interactions with a symmetrically located residue or enantiospecific interactions with different residues. The structures are color-coded according to atoms. Red, oxygen; blue, nitrogen; yellow, sulfur; gray, carbon; white, hydrogen.