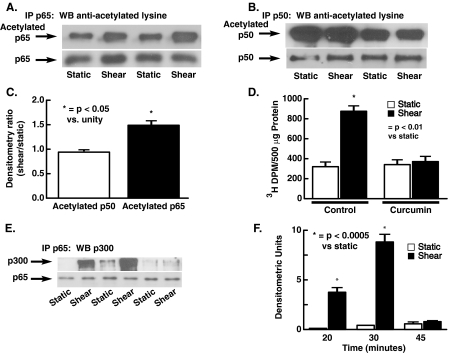
FIGURE 2.
Acetylation of p50 and p65 at base line and in response to laminar shear and association of p65 with p300. The NFκB subunits p50 and p65 were immunoprecipitated and Western blots using anti-lysine antibody performed. As shown in A and B, both p50 and p65 are acetylated at base line; however, 30 min of shear increases acetylation of p65 (A and C). C shows densitometric analysis of A and B, with the density of acetylated lysines in response to shear expressed as a ratio to the average static sample on the same blot. Shear (15 dynes/cm2 × 20 min) also increases acetyltransferase activity of p300, and this increase is prevented by the histone acetyltransferase inhibitor curcumin (80 mm in D, n = 3 for each). p300 was immunoprecipitated and acetyltransferase activity measured using histone substrate as described under “Experimental Procedures.” E shows the effect of shear on the association between p65 and p300. Mean data for densitometric analysis of three separate experiments are shown in F. Data are mean ± S.E. of the mean.