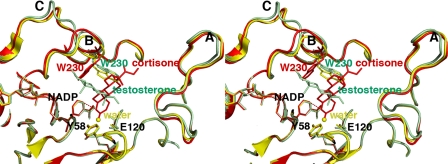
FIGURE 5.
Least-squares superposition of the AKR1D1·NADP+ (yellow), AKR1D1·NADP+·cortisone (red), and AKR1D1·NADP+·testosterone (green) complexes. The distance of ∼3.7 Å between the anomeric carbon of NADP+ and the olefinic C-4 of cortisone is indicated by a red dashed line, which would represent the trajectory of hydride transfer from NADPH. The position of the water molecule in the unliganded structure is indicated by a yellow sphere. Loops A, B, and C are labeled; note that loop B and Trp230 in particular must undergo a significant conformational change to accommodate productive substrate binding as represented by cortisone (red). The indole ring of Trp230 remains in its substrate-free conformation (yellow) to accommodate the nonproductive binding mode of testosterone (green).