FIG. 10.
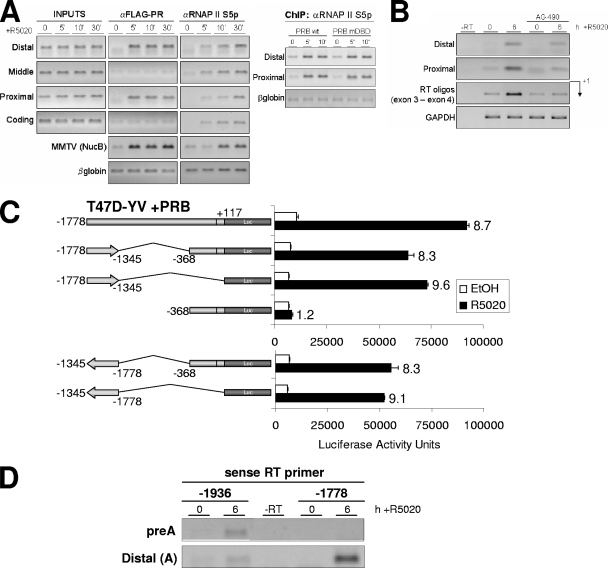
The distal region works as a hormone-responsive RNAP II entry site from where RNA synthesis occurs and enhances 11β-HSD2 expression. (A) TYML cells expressing WT FLAG-tagged PRB, cultured as described for Fig. 1A, were untreated (0) or treated with R5020 (10 nM) for 5 or 10 min, harvested, and used for ChIP experiments with anti-FLAG tag (αFLAG) and phospho(Ser5)-RNAP II (RNAP II S5p) antibodies. The precipitated DNA fragments were subjected to PCR analysis with specific primers corresponding to the indicated 11β-HSD2 promoter regions or MMTV nucleosome B and the β-globin gene as a control. On the right panel, anti-phospho-RNAP II ChIP was performed on chromatin extracted from TYML cells expressing FLAG-tagged WT PRB or PRB-mDBD, untreated or treated with R5020. (B) TYML cells expressing FLAG-tagged WT PRB, cultured as described for Fig. 1A, were treated with ethanol or R5020 (10 nM) for 6 h. When indicated, cells were pretreated with AG (50 μM). Cells were harvested, total RNA was prepared, and cDNA was generated by RT using oligo(dT). RNA synthesis was analyzed using primers that specifically amplified the 11β-HSD2 promoter distal region and the proximal region and primers located in exon 3 and exon 4 (RT oligos). GAPDH-specific primers were used as a control. (C) T47D-YV cells cotransfected with 1.5 μg of WT pSG5-PRB expression vector, 1.5 μg of the indicated 11β-HSD2 deletion constructs was treated with ethanol (EtOH) or R5020 (10 nM) for 16 h, and Luc activity was measured. For normalization, equal amounts of cellular extract of each sample were used. The values represent the mean numbers of arbitrary Luc units and ranges of a representative experiment performed in duplicate. Inductions in response to hormone are indicated. (D) TYML cells expressing FLAG-tagged WT PRB, cultured as described for Fig. 1A, were treated with EtOH or R5020 (10 nM) for 6 h. Then, cells were harvested, total RNA was prepared, and cDNA was generated by RT using gene-specific sense primers at positions −1936 and −1778 upstream of the TSS. The presence of antisense RNA upstream of the TSS was analyzed using PCR primers at positions −1936/−1779 (preA) and −1778/−1596 (distal A) that specifically amplified the promoter region.