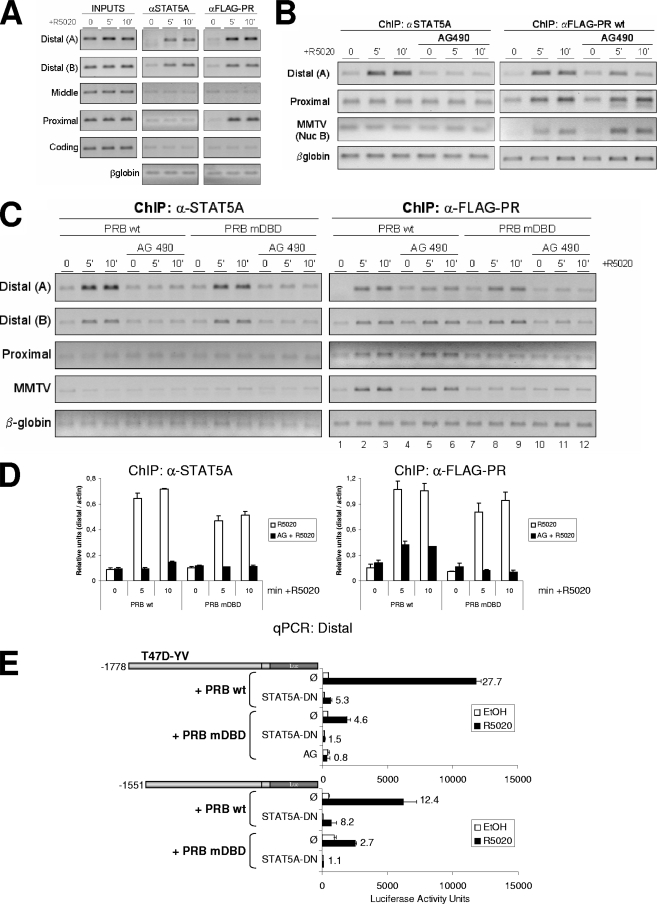
FIG. 8.
STAT5A and PR recruitment to the distal region depends on the JAK/STAT pathway. (A) TYML cells expressing FLAG-tagged WT PRB were cultured as described for Fig. 1A, untreated (0) or treated with R5020 (10 nM) for 5 or 10 min, harvested, and used for ChIP experiments using anti-STAT5A (αSTAT5A; middle panel) or anti-FLAG tag (right panel) antibodies. The precipitated DNA fragments were subjected to PCR analysis with primers corresponding to the indicated 11β-HSD2 promoter regions and the β-globin gene. Input material (1%) is shown for comparison. PCR products were run in a 1.2% agarose gel and visualized with ethidium bromide. (B) TYML cells expressing FLAG-tagged WT PRB, cultured as described for Fig. 1A, were untreated (0) or treated with R5020 (10 nM) for 5 or 10 min, harvested, and used for ChIP experiments with anti-STAT5A or anti-FLAG-tag antibodies. When indicated, cells were treated with AG (50 μM) 1 h before hormone addition. The precipitated DNA fragments were subjected to PCR analysis with specific primers corresponding to the distal and proximal 11β-HSD2 promoter regions or MMTV nucleosome B and the β-globin gene as a control. PCR products were run in a 1.2% agarose gel and visualized with ethidium bromide. (C) ChIP experiment as in panel B from TYML cells expressing FLAG-tagged WT PRB or mutant DBD PRB (PRB-mDBD), combined with AG (50 μM). Here, the average chromatin fragment size was 200 bp (see Materials and Methods). (D) Real-time PCR quantification of ChIP samples from panel C for distal amplicon A, performed in duplicate and normalized to actin gene amplification levels. The values represent the means and data ranges. (E) T47D-YV cells cotransfected with 1 μg of the 11β-HSD2 −1778 or −1551 promoter construct, 1 μg of WT or PRB-mDBD, and 1 μg of DN STAT5A when indicated were treated with ethanol (EtOH) or R5020 (10 nM) for 16 h, and Luc activity was measured. Where indicated, 1 h of AG pretreatment was performed. Induction in response to hormone is shown for each construct. The values represent the means and ranges of a representative experiment performed in duplicate.