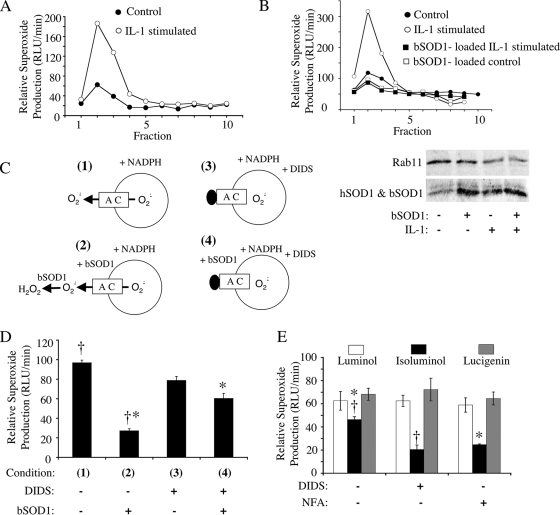
FIG. 1.
IL-1β-stimulated endosomes produce NADPH-dependent intravesicular ·O2− that can permeate endosomal membranes through a DIDS- and NFA-sensitive pathway. MCF-7 cells were stimulated with IL-1β (5 ng/ml) or vehicle for 20 min. The cells were lysed, and PNSs were fractionated on iodixanol gradients. (A) Fractions were evaluated for NADPH-dependent ·O2− production using a lucigenin-based luminescence assay. (B) MCF-7 cells were treated as described for panel A except that control and IL-1β stimulation were done in the presence or absence of 1 mg/ml SOD1 added to the medium at the time of stimulation. PNSs were fractionated on iodixanol gradients, and Western blotting (lower panel) was performed on the endosomal peak fraction (fraction 2) using antibodies against SOD1 and Rab11 (general endosomal marker). Results of one representative experiment of three are shown in panels A and B. (C) Strategy for assessing NADPH-dependent ·O2− flux (D) using a lucigenin-based luminescence assay on peak endosomal fraction 2 from IL-1β-stimulated cells. In these studies, SOD1 (0.01 mg/ml) and/or DIDS (100 μM) was added to the outside of isolated endosomes prior to adding NADPH to initiate ·O2− production. (D) The relative rate of ·O2− production under the conditions shown in panel C. Results are means and standard errors of the means from three experiments. (E) Comparison of NADPH-dependent ·O2− production levels by peak endosomal fraction 2 from IL-1β-stimulated cells in the presence of membrane-permeable (lucigenin and luminol) and membrane-impermeable (isoluminol) luminescent probes. Assays were performed in the presence or absence of 100 μM DIDS or NFA. Results are means and standard errors of the means from three experiments. Statistical differences obtained with Student's t test (P < 0.005) are indicated (*, †). RLU, relative light units.