Scheme 2.
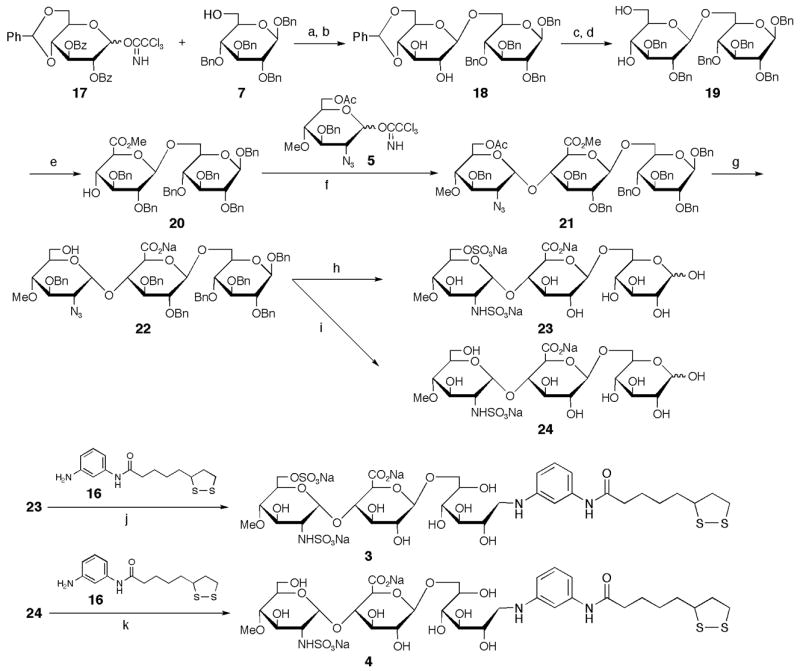
Synthesis of ligand conjugates 3 and 4 containing GlcNS6S-GlcA and GlcNS-GlcA, respectively. (a) BF3•OEt2, MS4AP in CH2Cl2, −20°C; (b) 0.1 M NaOMe, 90% (2 steps); (c) NaH, BnBr in DMF, 0°C→rt, 88%; (d)16% TFA, 8% MeOH in CH2Cl2, 0°Crt, 93%; (e) TEMPO, KBr, NaClO in CH2Cl2; TMSCHN2, 83% (2 steps); (f) TBDMSOTf, MS4AP in toluene, −20°C, 84%; (g) 5 M NaOH in MeOH/THF (1:1), 89%; (h) SO3•Pyr in Pyr; 10% Pd-C, H2 (1 kg/cm2) in THF/H2O (2:1); SO3•Pyr in H2O (pH 9.5); 10% Pd-C, H2 (7 kg/cm2) in H2O/AcOH (5:1), 28% (4 steps); (i) 10% Pd-C, H2 (1 kg/cm2) in THF/H2O (2:1); SO3•Pyr in MeOH/H2O (3:2); 10% Pd-C, H2 (7 kg/cm2) in H2O/MeOH/AcOH (5:5:2), 39% (3 steps); (j) NaBH3CN in DMAc/H2O/AcOH (1:1:0.1), 62%; (k) NaBH3CN in DMAc/H2O/AcOH (1:1:0.1), 50%.
