Abstract
We compared the outcomes of 141 consecutive patients who received allogeneic transplantation with either myeloablative (MA) or nonmyeloablative/reduced intensity (NMA) conditioning for non-Hodgkin and Hodgkin lymphoma at the University of Minnesota. All patients were transplanted between 1997 and 2004. NMA transplant recipients were older and received umbilical cord blood grafts more frequently (MA: 6 [9%]; NMA: 33 [43%], P < .001). NMA patients had more advanced disease and 30 (39%) patients had undergone prior autologous transplantation. The 4-year overall survival (OS) (MA: 46% versus NMA: 49%; p = .34) and the 3-year progression-free survival (PFS) (MA: 44% versus NMA: 31%; P = 0.82) were similar after MA or NMA conditioning. However, MA conditioning resulted in significantly higher 1-year treatment-related mortality (TRM) (MA: 43% versus NMA: 17%; P < .01) but a lower risk of relapse at 3 years (MA: 11% versus NMA: 36%; P < .01). We conclude that similar transplant outcomes are achieved after allogeneic hematopoietic stem cell transplantation using MA conditioning in younger patients and NMA conditioning in older patients or those with prior autologous transplantation not eligible for MA conditioning. Modifications to refine patient assignment to the preferred conditioning intensity and reduce relapse risks with NMA approaches are needed.
Keywords: Lymphoma, Allogeneic transplantation, Nonmyeloablative
INTRODUCTION
Lymphoma, particularly non-Hodgkin lymphoma (NHL), is progressively increasing in incidence [1]. Since 1995, autologous hematopoietic stem cell transplantation (AuHCT) has been the preferred management for diffuse large cell NHL in second remission [2]. The application of AuHCT to other lymphoma subtypes and prognostic indications defining the optimal timing of transplant continue to evolve. Despite this, nearly 20,000 persons in the United States are estimated to die from lymphoma in 2007, whereas only approximately 2500 autologous and allogeneic transplants per year are performed for lymphoma [1,3].
Relapse remains the most common cause of death after AuHCT for lymphoma [3]. Allogeneic transplant (AlloHCT) offers disease control from a graft-versus-lymphoma (GVL) effect in addition to control from the conditioning regimen, but is associated with higher treatment-related complications [4-7]. Continued improvements in the outcomes of AlloHCT and newer conditioning regimens have led to greater exploration of AlloHCT for patients with advanced lymphoma. Limited yet inconclusive data have been published comparing myeloablative (MA) versus nonmyeloablative (NMA) regimens for lymphoma management [8-11]. To better address the comparative safety and utility of conditioning intensity, we reviewed 141 consecutive patients with either NHL or Hodgkin lymphoma (HL) treated with AlloHCT in our institution.
METHODS
The University of Minnesota maintains a database of all consecutive patients enrolled in institutional review board-approved clinical transplantation trials. Retrospective review of this database identified all patients with either NHL or HL that were treated with AlloHCT between January 1997 and December 2004.
Eligibility
All patients had chemotherapy-responsive disease defined as achieving at least a minimal response to the preceding salvage regimen. Indications for NMA conditioning included at least 1 of the following: prior AuHCT; older age (>55 years for related donor or >45 years for an unrelated donor including umbilical cord blood); extensive prior therapy defined as >12 months of alkylator therapy or 6 months of alkylator therapy with extensive radiation; impaired cardiac or pulmonary function (ejection fraction ≥35% and/or corrected DLCO ≥30%, respectively); or recent fungal infection controlled with a minimum of 30 days of therapy. To be eligible for MA conditioning, patients were required to have an ejection fraction ≥45% and a corrected DLCO ≥50% and not have any of the other criteria indicating the need for NMA conditioning. Donors were either related donors (RD) (6 of 6 or 5 of 6 HLA match), adult volunteer unrelated donors (URD) (7-8 of 8 HLA match), or unrelated umbilical cord blood (UCB) units (4-6 of 6 HLA match). UCB transplants were either single or double units as previously described [12,13]. Patients seropositive for human immunodeficiency virus were excluded.
Treatment
MA conditioning (Table 1) consisted of intravenous (i.v.) cyclophosphamide (Cy) 120 mg/kg divided over 2 days plus either fractionated total-body irradiation (TBI) 1320 cGy divided twice daily over 4 days; or oral busulfan (Bu) 16 mg/kg divided every 6 hours over 4 days; or, for UCB transplants, TBI 1320 cGy plus i.v. fludarabine (Flu) 75 mg/m2 divided over 3 days. NMA conditioning consisted of a single fraction of TBI 200cGy along with either i.v. Flu 200 mg/m2 divided over 5 days plus i.v. Cy 50 mg/kg as a single dose; or i.v. Flu 200 mg/m2 plus oral Bu 8 mg/kg divided every 6 hours over 2 days; or i.v. Cladrabine 50 mg/m2 plus oral Bu 8 mg/kg. Equine antithymocyte globulin (15 mg/kg i.v. every 12 hours for 6 doses) was added to NMA conditioning if patients had not received any combination chemotherapy in the preceding 3 months for UCB (n = 2) or preceding 6 months (n = 2) for sibling transplants. Graft-versus-host disease (GVHD) prophylaxis was i.v. or oral cyclosporine (CSA) targeted to 200-400 ng/mL and either i.v. Methotrexate 15 mg/m2 on day +1 and 10 mg/m2 on days +3, +6, and +11 for MA conditioning; or i.v. or oral Mycophenylate mofetil (MMF) 1000 mg every 12 hours until day +30 after NMA conditioning or for recipients of UCB transplants regardless of conditioning intensity. All patients received antibacterial, antiviral, and antifungal prophylaxis and blood product support per institutional guidelines. All patients received filgrastim posttransplant until achieving an absolute neutrophil count (ANC) ≥2.5 × 109/L for 2 days.
Table 1. Patient Characteristics Compared by Conditioning Cohorts.
| Myeloablative |
Nonmyeloablative |
||
|---|---|---|---|
| N = 65 |
N = 76 |
||
| N (%) | N (%) | P-value | |
| Age, years-median (range) | 42 (4-58) | 48 (19-66) | <.01 |
| Gender, male | 40 (62) | 46 (61) | NS |
| Disease | |||
| NHL* | 62 (95) | 53 (70) | <.01 |
| HL | 3 (5) | 23 (30) | |
| Disease status at transplant | |||
| CR1+ | 18 (28) | 11 (14) | NS |
| PR1+ | 37 (57) | 49 (64) | |
| Minimally responsive disease | 10 (15) | 16 (21) | |
| Prior autologous transplant | |||
| Yes | 0 | 30 (39) | <.01 |
| No | 65 (100) | 46 (61) | |
| Donor | |||
| Matched related | 49 (75) | 32 (42) | <.01 |
| Unrelated/mismatched related | 10 (15) | 11 (14) | |
| Umbilical cord blood | 6 (9) | 33 (43) | |
| Stem cell source | |||
| Bone marrow | 5 (8) | 8 (10) | <.01 |
| PBSC | 54 (83) | 35 (46) | |
| Umbilical cord blood | 6 (9) | 33 (43) | |
| Donor/recipient CMV serostatus | |||
| Both negative | 21 (32) | 40 (53) | .02 |
| Either positive | 44 (68) | 36 (47) | |
| Conditioning regimen† | |||
| Cy120/TBI (1320 cGy) | 49 (75) | ||
| Bu16/Cy120 | 12 (18) | ||
| Flu75/Cy120/TBI (1320 cGy) | 4 (6) | ||
| Flu200/Cy50/TBI (200 cGy) | 48 (64) | ||
| Flu200/Bu8/TBI (200 cGy) | 21 (27) | ||
| Bu8/Clad50/TBI (200 cGy) | 7 (9) | ||
| GVHD prophylaxis | |||
| CSA/Methotrexate | 59 (91) | 0 | <.01 |
| CSA/MMF | 6 (9) | 76 (100) | |
| Year of transplant | |||
| 1997-2000 | 31 (48) | 10 (13) | <.01 |
| 2001-2004 | 34 (52) | 66 (87) | |
| Time from diagnosis to transplant (month) | 16 (3-106) | 30 (8-247) | <.01 |
GVHD indicates graft-versus-host disease; PBSC, peripheral blood stem cells; NHL, non-Hodgkin’s lymphoma; CR, complete remission; PR, partial remission; CMV, cytomegalovirus; HL, Hodkin’s lymphoma; TBI, total-body irradiation.
Mantle cell lymphoma (MA, n = 13; NMA, n = 7); indolent lymphomas including follicular and small lymphocytic lymphoma (MA, n = 11; NMA, n = 24); diffuse large cell lymphoma (MA, n = 7; NMA, n = 11); T cell lymphomas (MA, n = 8; NMA, n = 6); and other lymphomas including aggressive lymphomas such as transformed or Burkitt’s lymphomas (MA, n = 23; NMA, n = 5).
Cy120 = cyclophosphamide 120 mg/kg total dose; Cy50 = Cyclophosphamide 50 mg/kg total dose; Bu16 = Busulfan 16 mg/kg total dose; Bu8 = Busulfan 8 mg/kg total dose; Flu75 = Fludarabine 75 mg/m2total dose; Flu200 = Fludarabine 200 mg/m2total dose; Clad50 = Cladrabine 50 mg/m2 total dose.
Statistical Analysis
Patient and transplant characteristics by type of conditioning were analyzed using the chi-square test for categoric data and the Wilcoxon Rank-Sum test for continuous data. The primary study endpoint was overall survival (OS). Secondary endpoints were progression-free survival (PFS), treatment-related mortality (TRM), relapse or disease progression, acute GVHD (aGVHD) grade II-IV and grade III-IV, chronic GVHD (cGVHD), neutrophil engraftment, and platelet engraftment.
Event time for neutrophil engraftment was the date of transplantation to the first of 3 consecutive days with an ANC above 0.5 × 109/L. The cumulative incidence of neutrophil engraftment was calculated by treating patients without an ANC >0.5 × 109/L at day 42 or with autologous marrow reconstitution as primary graft failures. Time to platelet engraftment was defined as the first day with a platelet count >20 × 109/L without transfusions for the 7 following days.
Diagnosis of aGVHD and cGVHD was based on standard clinical criteria with histopathologic confirmation where possible [14]. The cumulative incidences of neutrophil and platelet engraftment, aGVHD, and cGVHD and relapse were calculated by treating deaths from other causes as competing risks [15]. OS and PFS were estimated by the Kaplan-Meier method [16]. PFS was defined at the time of last follow-up for those patients that were alive without disease relapse or progression. TRM was defined as death without disease progression or relapse. Diagnoses of disease response and relapse or progression were defined according to standard criteria for lymphoma [17]. Event times were measured from the date of transplantation to the event or the date of last contact. Statistical comparisons of the time-to-event curves were completed by the log-rank test or the Gray method, where appropriate.
Proportional hazards regression modeling was used for multiple regression analysis with Cox regression and the Gray and Fine competing hazards method as appropriate [18,19]. Variables considered in the models included the main effect variable of conditioning intensity (MA versus NMA) along with age (≤45 years versus >45 years), gender, donor type by HLA disparity (matched RD versus URD/mismatched RD versus UCB), CMV serostatus (donor and recipient negative versus either positive), year of transplant (1997-2000 versus 2001-2004), aGVHD as a time-dependent variable, diagnosis (NHL versus HL), and disease status at transplant (CR 1+ versus PR 1+ versus minimally responsive disease defined as less than PR but responsive to the most recent therapy). Because of small numbers within each pathologic subtype of NHL, histology was not included as a variable in the comparison of outcomes after MA and NMA conditioning. Factors were included in the model if marginal significance (P < .1) was noted. All factors satisfied the proportional hazards assumptions.
RESULTS
Patient Characteristics
From 1997-2004, 141 consecutive patients with either NHL (n = 115) or HL (n = 26) were treated. Patient characteristics according to conditioning intensity (MA, n = 65; NMA, n = 76) are summarized in Table 1. Significantly more NMA patients were older, were transplanted more recently, and were more likely to have received UCB. Similar percentages of patients received marrow grafts in each group. Of 39 patients receiving UCB, 26 patients (67%; MA, n = 2; NMA, n = 24) received 2 UCB units. Only patients in the NMA cohort had undergone previous AuHCT. Older age was the predominant reason for NMA conditioning (n = 48) and the median age for patients without prior AuHCT was 52 years (23-62 years). Patients younger than 45 years of age (n = 28) received NMA conditioning because of prior AuHCT (n = 17) or extensive prior therapy (n = 11). The median follow-up of survivors for each cohort was similar (MA: 39 months [range: 23-106]; NMA: 36 months [range: 12-56]).
Survival
OS for the entire cohort at 4 years was 48% (95% confidence interval [CI]: 39-56), with no difference between conditioning regimen intensity (4-year OS: MA: 46% [95% CI: 34-59] versus NMA: 49% [95% CI: 37-61]; P = .34) (Figure 1A). In univariate analysis, the use of URD/mismatched RD compared to HLA-identical RD was the only significant factor, and remained significant in multiple regression analysis (relative risk [RR] 2.9 [95% CI 1.6-5.3, P < .01) (Table 2). Conditioning intensity, disease status at transplant, diagnosis, and the use of UCB did not significantly impact OS.
Figure 1.
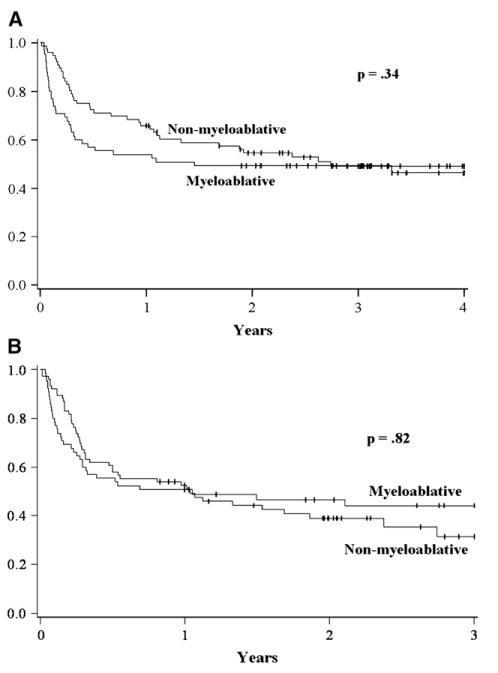
Unadjusted Kaplan-Meier estimates of overall survival (A) and progression-free survival (B) comparing myeloablative and NMA conditioning.
Table 2. Multivariate Analysis* for Survival, Progression-Free Survival, and Relapse/Progression.
| Overall Survival | ||
|---|---|---|
| Relative Risk of Death (95% CI) | P-Value | |
| Conditioning regimen | ||
| Myeloablative | 1.0 | |
| Nonmyeloablative | 0.7 (0.4-1.1) | .14 |
| Donor type | ||
| Matched related | 1.0 | |
| Unrelated/mismatched related | 2.9 (1.6-5.3) | <.01 |
| Umbilical cord blood | 1.4 (0.8-2.6) | .41 |
| Progression-Free Survival | ||
|---|---|---|
| Relative Risk of Death or Progression/Relapse (95% CI) | P-Value | |
| Conditioning regimen | ||
| Myeloablative | 1.0 | |
| Nonmyeloablative | 0.7 (0.4-1.2) | .24 |
| Donor type | ||
| Matched related | 1.0 | |
| Unrelated/mismatched related | 2.4 (1.4-4.4) | <.01 |
| Umbilical cord blood | 1.4 (0.8-2.4) | .29 |
| Diagnosis | ||
| NHL | 1.0 | |
| Hodgkins | 1.9 (1.1-3.2) | .03 |
| Disease status | ||
| CR1+ | 1.0 | |
| PR1+ | 0.8 (0.4-1.6) | .39 |
| Minimally responsive disease | 1.9 (0.9-4.1) | .09 |
| Relapse/Progression | ||
|---|---|---|
| Relative Risk of Relapse or Disease Progression (95% CI) | P-Value | |
| Conditioning regimen | ||
| Myeloablative | 1.0 | |
| Nonmyeloablative | 3.3 (1.2-9.2) | .03 |
| Donor type | ||
| Matched related | 1.0 | |
| Unrelated/mismatched related | 1.1 (0.4-3.2) | .88 |
| Umbilical cord blood | 2.1 (0.9-5.1) | .10 |
CI indicates confidence interval; NHL, non-Hodgkin’s lymphoma; CR, complete remission; PR, partial remission.
The above models are the result of multiple regression analysis after testing the following variables: age, weight, gender, donor type, CMV serostatus, acute GVHD (time-dependent variable), year of transplant (1997-2000 versus 2001-2004), diagnosis, and disease status. Conditioning intensity, as the main effect variable, is presented in every model. Otherwise, only factors with at least marginal significance are reported.
PFS at 3 years did not differ between the MA and NMA cohorts in either univariate or multiple regression analysis (Figure 1B and Table 2). At 3 years the PFS for MA conditioning was 44% (95% CI: 31-57) and NMA conditioning was 31% (95% CI: 18-49) (P = .82). By univariate analysis, PFS was inferior for patients receiving URD/mismatched RD and for patients with minimally responsive disease prior to transplant. There was a trend toward inferior PFS for patients with HL compared to NHL. In Cox regression, URD/mismatched RD and HL were associated with significantly inferior PFS. There remained a trend toward higher risk of death or progression for patients with minimally responsive disease (Table 3).
Table 3. Multiple Regression Analysis for Acute and Chronic GVHD (aGVHD, cGVHD).
| Grade II-IV aGVHD | ||
|---|---|---|
| Relative Risk of GVHD (95% CI) | P-Value | |
| Conditioning regimen | ||
| Myeloablative | 1.0 | |
| Nonmyeloablative | 1.3 (0.8-2.2) | .23 |
| Donor type | ||
| Matched related | 1.0 | |
| Unrelated/mismatched related | 1.6 (0.8-2.9) | .16 |
| Umbilical cord blood | 1.3 (0.7-2.2) | .38 |
| cGVHD | ||
|---|---|---|
| Relative Risk of GVHD (95% CI) | P-Value | |
| Conditioning regimen | ||
| Myeloablative | 1.0 | |
| Nonmyeloablative | 2.2 (1.3-3.9) | <.01 |
| Donor type | ||
| Matched related | 1.0 | |
| Unrelated/mismatched related | 0.9 (0.4-1.8) | .63 |
| Umbilical cord blood | 0.5 (0.3-0.9) | .03 |
CI indicates confidence interval; GVHD, graft-versus-host disease.
The 1-year TRM was 2.5 times higher after MA conditioning with a 1 year TRM of 43% (95% CI: 31-55) for the MA cohort and only 17% (95% CI: 9-25) in the NMA cohort (P = .05). In multivariate analysis, increased risk of TRM was associated with use of an URD/mismatched RD (RR: 2.1 [1.0-4.6], P = .05) and development of aGVHD ≥ grade II (RR: 18.4 [4.9-68.2], P < .01). The use of NMA conditioning decreased the risk of TRM (RR: 0.3 [0.2-0.7], P < .01).
Relapse and Disease Progression
NMA conditioning was associated with an increased risk of relapse or disease progression at 3 years compared to those patients receiving a MA regimen (MA: 11% [95% CI: 3-19] versus NMA: 36% [95% CI: 24-48], P < .01). Other factors predictive of relapse or disease progression by univariate analysis included the use of UCB compared to HLA-identical matched RD or URD/mismatched RD (42% [95% CI: 25-59] versus 17% [95% CI: 8-26] versus 19% [95% CI: 3-35]; P < .01). Patients not achieving a PR or CR immediately prior to transplant trended toward an increased risk of relapse or disease progression (at 1 year: minimally responsive disease 35% [95% CI: 16-54] versus PR 14% [95% CI: 7-21] versus CR 21% [95% CI: 6-36]; P = .08). In multiple regression analysis, NMA conditioning and UCB donor remained as increased risk factors for relapse or progression (Table 2). Disease status at transplant was not an independent risk factor for relapse or disease progression in the regression model.
GVHD
The median time to onset for aGVHD grade II-IV was slightly but not significantly longer for patients receiving NMA conditioning versus MA patients (MA: 29 days [14-73]; NMA: 35 days [14-86], P = .39). The cumulative incidence of grade II-IV aGVHD at day 100 was 51% (95% CI: 42-60) and was similar after MA and NMA conditioning (MA: 43% [95% CI: 30-56]; NMA: 58% [95% CI: 46-70]; P = .47). Grade III-IV aGVHD cumulative incidence was 20% (14-26%) and was not statistically different between MA (14% [95% CI: 6-22]) and NMA (25% [95% CI: 17-33]) conditioning (P = .26). Multiple regression analysis found that HCT using either an URD/mismatched RD resulted in a 2-fold, although not significantly increased risk of grade III-IV aGVHD (RR 2.0 [95% CI: 0.7-6.2]; P = .21]) (Table 3). Conditioning intensity or UCB as the stem cell source had no impact on the incidence or severity of aGVHD.
Chronic GVHD occurred in 46% (95% CI: 36-56) of patients by 2 years (MA: 35% [95% CI: 22-48] versus NMA: 55% [95% CI: 41-69]; P = .12]). Onset of cGVHD occurred at a median (range) of 185 days (80-727) after MA conditioning and at 167 days (69-514) after NMA conditioning (P = .47). Multiple regression analysis (Table 3) found that NMA recipients were more than 2-fold as likely to develop cGVHD (RR 2.2 [95% CI: 1.3-3.9]; P < 0.01). Patients receiving UCB stem cells were significantly less likely to develop cGVHD compared to HLA-identical RD (RR 0.5 [95% CI: 0.3-0.9]; P = .03).
Engraftment
The cumulative incidence of sustained neutrophil engraftment was 96% (95% CI: 92-99%). Neutrophil recovery occurred at a median of 13 days (range: 0-32) but was significantly faster in patients receiving a NMA conditioning regimen (MA: 16 days [range, 11-31] versus NMA 10 days [0-32]; P < 0.01). Platelet engraftment by 6 months occurred in 81% (95% CI: 70-92) of patients at a median of 26 days (range: 0-134). Platelet engraftment was more rapid after NMA conditioning (MA: 28 days [range: 16-134] versus NMA: 19 days [range; 0-69], P = .02).
Prior Autologous Transplant
Thirty patients (39%) in the NMA cohort had relapsed after a prior autologous transplant. We analyzed the outcomes of this subset of patients in comparison to those in the NMA cohort who had not received a prior autologous transplant. The transplant-related outcomes of relapse, TRM, PFS, and OS were similar between patients conditioned with a NMA regimen regardless of prior autologous transplant (Table 4).
Table 4. Transplant Outcomes in the NMA Cohort Comparing Patients with and without a Prior Autologous HCT (AuHCT).
| Prior AuHCT (n = 30) Estimate (95% CI) | No prior AuHCT (n = 46) Estimate (95% CI) | P-Value | |
|---|---|---|---|
| 3-year OS | 48% (33-63) | 54% (35-73) | .82 |
| 3-year PFS | 30% (12-51) | 36% (21-50) | .86 |
| 3-year relapse/progression | 28% (13-43) | 40% (25-55) | .35 |
| 1-year TRM | 23% ( 8-38) | 13% ( 3-23) | .43 |
OS indicates overall survival; PFS, progression-free survival; TRM, treatment-related mortality.
Prognostic factors Following NMA Conditioning
We performed an exploratory analysis to better identify the patients who will experience improved outcomes after NMA conditioning. The heterogeneity of NHL resulted in small numbers of each pathologic subtype although adequate patients (n = 24) with low-grade lymphomas (follicular [FL] and small lymphocytic lymphoma [SLL]) were available for subgroup analysis. Factors analyzed included histology (FL/SLL versus HL versus other NHL), disease status at transplant (CR versus PR versus minimally responsive), stem cell source (MRD versus URD/mismatched RD versus UCB), time from diagnosis to transplant (≤1 year versus >1-2 years versus ≥2 years), development of aGVHD grade II-IV and the development of cGVHD. Univariate analysis for PFS showed a trend toward improved PFS at 3 years for patients with FL/SLL (FL/SLL: 53% [95% CI: 29-77] versus HL: 20% [95% CI: 13-37] versus other NHL: 29% [95% CI: 9-49]; P = .08) (Figure 2). The cumulative incidence of relapse was similar regardless of histology (FL/SLL: 36% [95% CI: 14-58] versus HL: 25% [95% CI: 15-55] versus other NHL: 37% [95% CI: 19-55]; P = .9). The trend toward improved PFS in the FL/SLL group was due primarily to lower 1-year TRM (FL/SLL: 4% [95% CI: 0-12] versus HL: 26% [95% CI: 8-44] versus other NHL: 20% [95% CI: 6-34]). In Cox regression analysis (Table 5), FL/SLL had significantly decreased risk of relapse or death compared to HL and other NHL (FLL/SLL: RR 0.4 [95% CI: 0.1-0.9] versus HL: RR 1.0 versus other NHL: RR 0.7 [95% CI: 0.3-1.3]; P = .02). Similar outcomes were observed for patients either in CR or PR, but were inferior for minimally responsive disease (RR 2.7 [95% CI: 1.0-7.4], P = .05). Improved survival was noted for patients 2 or more years from diagnosis (RR 0.3 [95% CI: 0.1-0.6], P < .01). Neither aGVHD nor cGVHD were prognostic, and no factors predicted relapse after NMA conditioning by univariate or multivariate analysis. For the cohort of NMA patients (n = 12) with FL/SLL in CR or PR transplanted 2 or more years from diagnosis, the 3-year PFS was 83% (95% CI: 62-100).
Figure 2.
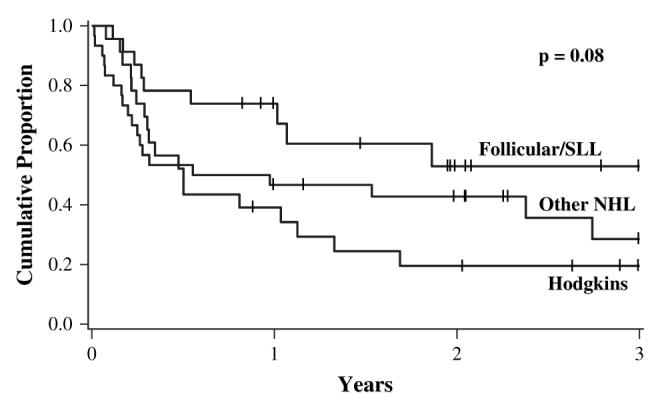
Unadjusted Kaplan-Meier estimates for progression-free survival after NMA conditioning by histologic cohorts. Follicular/small lymphocytic lymphoma (SLL) (n = 24), Hodgkins (n = 23), other NHL (n = 29).
Table 5. Multiple Regression Analysis for PFS following NMA Conditioning.
| Progression-Free Survival (PFS) after NMA Conditioning | ||
|---|---|---|
| Relative Risk of Death without Progression/Relapse (95% CI) | P-Value | |
| Histologic diagnosis | ||
| Hodgkins | 1.0 | |
| FL/SLL | 0.4 (0.1-0.9) | .02 |
| Other NHL | 0.7 (0.3-1.3) | .24 |
| Disease status | ||
| CR1+ | 1.0 | |
| PR1+ | 1.2 (0.5-2.9) | .68 |
| Minimally responsive disease | 2.7 (1.0-7.4) | .05 |
| Years from diagnosis to HCT | ||
| ≤ year | 1.0 | |
| 1-<2 years | 0.4 (0.2-1.2) | .10 |
| ≥2 years | 0.3 (0.1-0.6) | <.01 |
NHL indicates non-Hodgkin’s lymphoma; CR, complete remission; PR, partial remission; HCT, hematopoietic cell transplants; CI, confidence interval; FL, follicular; SLL, small lymphocytic lymphoma.
DISCUSSION
Our analysis demonstrates similar OS and PFS after AlloHCT following either MA or NMA conditioning. Older patients, heavily pretreated patients, and patients who relapsed after a prior AuHCT comprised the bulk of the patients in the NMA cohort. The reasons for failure differed with higher TRM after MA conditioning balanced by the greater risk of relapse after NMA conditioning. There was no impact of prior AuHCT on outcomes. Both HLA-identical RD and UCB grafts were suitable for either MA or NMA transplants.
Four retrospective analyses have compared MA to NMA conditioning for lymphoma with varied out-comes [8-11]. In a small series (n = 23), Bertz and colleagues [8] found improved 1-year OS after Flubased NMA conditioning (67%) compared to MA conditioning (23%, P < .02). Rodriguez et al [9] reported on 88 patients (matched RD, n = 63; URD, n = 25) transplanted between 1991 and 2003. The MA and NMA cohorts were sequential as the center changed from MA conditioning to NMA conditioning for lymphoma patients in 2000. Similar to our study, they found no difference in OS and PFS at 2 years based on conditioning intensity. An analysis of 168 patients with HL from the European Blood and Marrow Transplantation Group (EBMT) [11] noted a trend toward improved 5-year OS after NMA conditioning (28% [95% CI: 18-38]) compared to MA conditioning (22% [95% CI: 13-31%]). Multivariate analysis demonstrated a 2-fold relative risk of decreased survival after MA conditioning. Sorror et al. [10,20] reported that improved OS and lower TRM is only realized in those patients with an HCT-specific comorbidity index score of ≥1 receiving an NMA conditioning regimen (score 1-2: n = 46; score 3: n = 62) compared to MA conditioning (score 1-2: n = 18; score 3: n = 22). Similar to our study, all of these analyses are limited by significant differences in patient characteristics because of selection bias for conditioning intensity based on center specific criteria. Despite this limitation, the data from our study and others suggest that NMA conditioning in older or more heavily pretreated patients is a reasonable therapeutic option.
The heterogeneity of histologic subtypes and disease status in lymphoma confounds outcomes assessment after AlloHCT. An analysis from the EBMT evaluated 188 lymphoma patients after NMA conditioning with a short median follow-up (<1 year) [21]. The estimated 1-year OS and PFS were 62% and 46%, respectively. The EBMT study found that resistant lymphoma or high grade lymphoma yielded inferior PFS. They reported no effect of donor type on transplant outcomes. In our study, only the use of a mismatched RD/URD or a diagnosis of HL led to decreased PFS. Within the exploratory analysis after NMA conditioning, factors associated with improved PFS included an indolent histology, pretransplant CR or PR, and 2 or more years from diagnosis until transplant. Our data, in conjunction with the EBMT report, suggests that the graft-versus lymphoma effect may be most potent in slow growing and responsive disease. Our outcomes after NMA conditioning are similar to other studies reported [22,23].
In multivariate analysis, we demonstrated a 2-fold increased risk of cGVHD after NMA transplantation compared to MA transplantation. This is somewhat unexpected given that UCB transplant was associated with a lower risk of cGVHD and the majority of UCB recipients received NMA conditioning. Possibilities include the differences in graft source, HLA matching, and GVHD prophylaxis that were confounded by the main effect variable of conditioning intensity. However, our incidence of cGVHD after NMA conditioning is similar to other reports of NMA conditioning for lymphoma [22,23]. We also noted a trend toward an increased risk of relapse in patients receiving UCB transplant. This may be correlated with the 2-fold reduction in cGVHD for UCB recipients. This suggests that there may be a correlation between cGVHD and the GVL effect.
Retrospective studies comparing MA and NMA conditioning are confounded by differences in patient characteristics for those who receive either conditioning intensity. Our institutional algorithm dictates conditioning intensity based upon age, extent of prior therapy and comorbidity. The heterogeneity of the lymphomas would require that any prospective studies be designed to study the impact of conditioning intensity with attention to specific histologic subtypes and careful identification of patients suitable for each conditioning approach. A Blood and Marrow Transplant Clinical Trials Network (BMT CTN)-Cooperative Intergroup Study is in development to assess the outcomes of NMA AlloHCT in patients with Follicular NHL, a histologic subgroup where AlloHCT has shown promise [24,25].
We observe that similar transplant outcomes are achieved after AlloHCT with MA conditioning in younger patients compared to NMA conditioning in older patients or those with prior AuHCT. Prospective trials studying NMA conditioning in specific histologic subtypes appropriate for patient age and relevant comorbidities are warranted to define the best application of AlloHCT for NHL.
REFERENCES
- 1.SEER Program (National Cancer Institute [U.S.]) National Institutes of Health (U.S.), National Cancer Institute (U.S.). Surveillance Program. SEER Cancer Statistics Review [Google Scholar]
- 2.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333:1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 3.CIBMTR Summary Slides http://www.cibmtr.org/SERVICES/Observational_Research/Summary_Slides/index.html.
- 4.Verdonck LF, Dekker AW, Lokhorst HM, Petersen EJ, Nieuwenhuis HK. Allogeneic versus autologous bone marrow transplantation for refractory and recurrent low-grade non-Hodgkin’s lymphoma. Blood. 1997;90:4201–4205. [PubMed] [Google Scholar]
- 5.van Besien K, Loberiza FR, Jr., Bajorunaite R, et al. Comparison of autologous and allogeneic hematopoietic stem cell transplantation for follicular lymphoma. Blood. 2003;102:3521–3529. doi: 10.1182/blood-2003-04-1205. [DOI] [PubMed] [Google Scholar]
- 6.van Besien K, Sobocinski KA, Rowlings PA, et al. Allogeneic bone marrow transplantation for low-grade lymphoma. Blood. 1998;92:1832–1836. [PubMed] [Google Scholar]
- 7.Peggs KS, Hunter A, Chopra R, et al. Clinical evidence of a graft-versus-Hodgkin’s lymphoma effect after reduced-intensity allogeneic transplantation. Lancet. 2005;365:1934–1941. doi: 10.1016/S0140-6736(05)66659-7. [DOI] [PubMed] [Google Scholar]
- 8.Bertz H, Illerhaus G, Veelken H, Finke J. Allogeneic hematopoetic stem-cell transplantation for patients with relapsed or refractory lymphomas: comparison of high-dose conventional conditioning versus fludarabine-based reduced-intensity regimens. Ann Oncol. 2002;13:135–139. doi: 10.1093/annonc/mdf010. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez R, Nademanee A, Ruel N, et al. Comparison of reduced-intensity and conventional myeloablative regimens for allogeneic transplantation in non-Hodgkin’s lymphoma. Biol Blood Marrow Transplant. 200612;12:1326–1334. doi: 10.1016/j.bbmt.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 10.Sorror ML, Storer BE, Maloney DG, Sandmaier BM, Martin PJ, Storb R. Outcomes after allogeneic hematopoietic cell transplantation with nonmyeloablative or myeloablative conditioning regimens for treatment of lymphoma and chronic lymphocytic leukemia. Blood. 2008;111:446–452. doi: 10.1182/blood-2007-07-098483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sureda A, Robinson S, Canals C, et al. Reduced-intensity conditioning compared with conventional allogeneic stem-cell transplantation in relapsed or refractory Hodgkin’s lymphoma: an analysis from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2008;26:455–462. doi: 10.1200/JCO.2007.13.2415. [DOI] [PubMed] [Google Scholar]
- 12.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 13.Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplant outcomes in 110 adults with hematological disease. Blood. 2007;110:3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 15.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16:901. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457. [Google Scholar]
- 17.Cheson BD, Horning SJ, Coiffier B, et al. NCI Sponsored International Working Group Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 18.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187. [Google Scholar]
- 19.Fine JP, Gray RJ. A proportional hazard model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496. [Google Scholar]
- 20.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson SP, Goldstone AH, Mackinnon S, et al. Chemoresistant or aggressive lymphoma predicts for a poor outcome following reduced-intensity allogeneic progenitor cell transplantation: an analysis from the Lymphoma Working Party of the European Group for Blood and Bone Marrow Transplantation. Blood. 2002;100:4310–4316. doi: 10.1182/blood-2001-11-0107. [DOI] [PubMed] [Google Scholar]
- 22.Corradini P, Dodero A, Farina L, et al. Allogeneic stem cell transplantation following reduced-intensity conditioning can induce durable clinical and molecular remissions in relapsed lymphomas: pre-transplant disease status and histotype heavily influence outcome. Leukemia. 2007;21:2316–2323. doi: 10.1038/sj.leu.2404822. [DOI] [PubMed] [Google Scholar]
- 23.Vigouroux S, Michallet M, Porcher R, et al. Long-term outcomes after reduced-intensity conditioning allogeneic stem cell transplantation for low-grade lymphoma: a survey by the French Society of Bone Marrow Graft Transplantation and Cellular Therapy (SFGM-TC) Haematologica. 2007;92:627–634. doi: 10.3324/haematol.10924. [DOI] [PubMed] [Google Scholar]
- 24.Khouri IF, Saliba RM, Giralt SA, et al. Nonablative allogeneic hematopoietic transplantation as adoptive immunotherapy for indolent lymphoma: low incidence of toxicity, acute graft-versus-host disease, and treatment-related mortality. Blood. 2001;98:3595–3599. doi: 10.1182/blood.v98.13.3595. [DOI] [PubMed] [Google Scholar]
- 25.Khouri IF, Lee M, Saliba RM, et al. Non-myeloablative allogeneic transplantation (NMT) with T-cell replete graft for relapsed chemosensitive follicular lymphoma (FL): Donor lymphocyte infusion (DLI) to convert stable mixed chimerism to full donor chimerism is not necessary in the absence of disease progression. Blood. 2005;106:3659. [Google Scholar]


