Fig. 8.
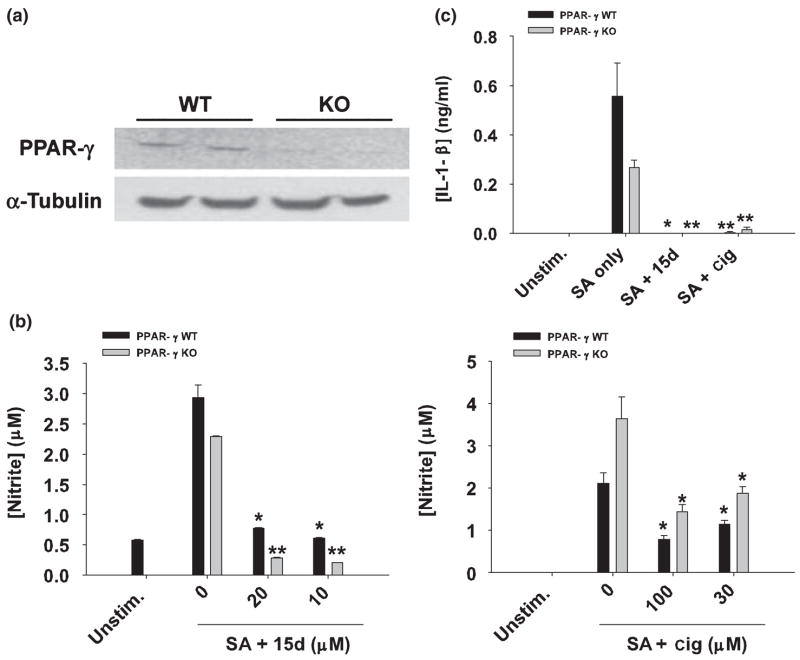
PPAR-γ null astrocytes are sensitive to the anti-inflammatory effects of 15d-PGJ2 and ciglitazone. (a) Primary astrocytes do not express full-length PPAR-γ mRNA. Primary astrocyte cultures were prepared from post-natal day 1 frontal cortices of astrocyte-conditionally null mice or wild type (WT) littermates as described in Materials and methods. After 2 weeks in culture, whole-cell RNA was isolated, converted to cDNA, and amplified using primers specific for PPAR-γ exon 2. Aliquots of the cDNA were also amplified using primers for α-tubulin to confirm RNA and cDNA integrity. The gels depict PCR products obtained from astrocyte RNA prepared from two individual WT and two PPAR-γ null (KO) mice. In (b), PPAR-γ WT and KO astrocytes were seeded in 6-well plates at 1 × 106 cells per well and incubated overnight. The following day, astrocytes were pre-incubated with 15d-PGJ2 (15d; 10–20 μM) or ciglitazone (cig; 30–100 μM) for 1 h and subsequently stimulated with 107 heat-inactivated S. aureus (SA). Cell-free supernatants were collected at 48 h following S. aureus treatment and analyzed for nitrite production (mean ± SD of four independent wells per treatment). In (c), PPAR-γ KO and WT astrocytes were pretreated with 15d-PGJ2 (20 μM) or ciglitazone (100 μM) for 1 h, followed by 107 heat-inactivated S. aureus (SA). Cell-free supernatants were collected 48 h following S. aureus treatment and IL-1β levels were quantified by ELISA. Results are reported as the mean ± SD of four independent wells for each experimental treatment. Significant differences between astrocytes treated with S. aureus only versus cells exposed to the various concentrations of 15d-PGJ2 + S. aureus or ciglitazone + S. aureus are denoted with asterisks (*p < 0.05, **p < 0.001). Results are representative of two independent experiments.