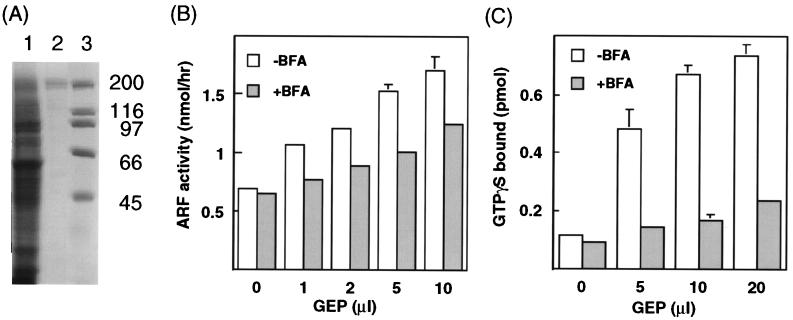
Figure 4.
(A) Purification of p200 synthesized as a six-His fusion protein in Sf9 cells. After separation by SDS/PAGE in 10% gel, proteins were stained with Coomassie blue. Lanes: 1, soluble proteins (30 μg) from lysate of induced Sf9 cells; 2, affinity-purified six-His-tagged p200 (≈100 ng); 3, standard proteins, size (kDa) on the right. (B) Effect of recombinant six-His-tagged p200 on ARF activity. ARF activity is CTA activity (nmol of ADP-ribosylagmatine synthesized per hr) with ARF minus that without ARF. Assays with the indicated amounts of purified six-His-tagged-p200 (≈3 μg/ml) were carried out without and with 6 μg of BFA. Data are means ± SEM of values of two samples. This experiment was replicated three times with different preparations. Activity of the partially purified ARF (10 μg) was equivalent to ≈0.1 μg of purified ARF3. (C) Effect of BFA on six-His-tagged p200 stimulation of GTPγS binding by ARF. Assays with the indicated amount of purified six-His-tagged-p200 (≈3 μg/ml) and 10 μg of partially purified native ARFs without or with 6 μg of BFA were incubated for 20 min at 30°C. Data are means ± SEM of values from triplicate assays. This experiment was replicated twice.