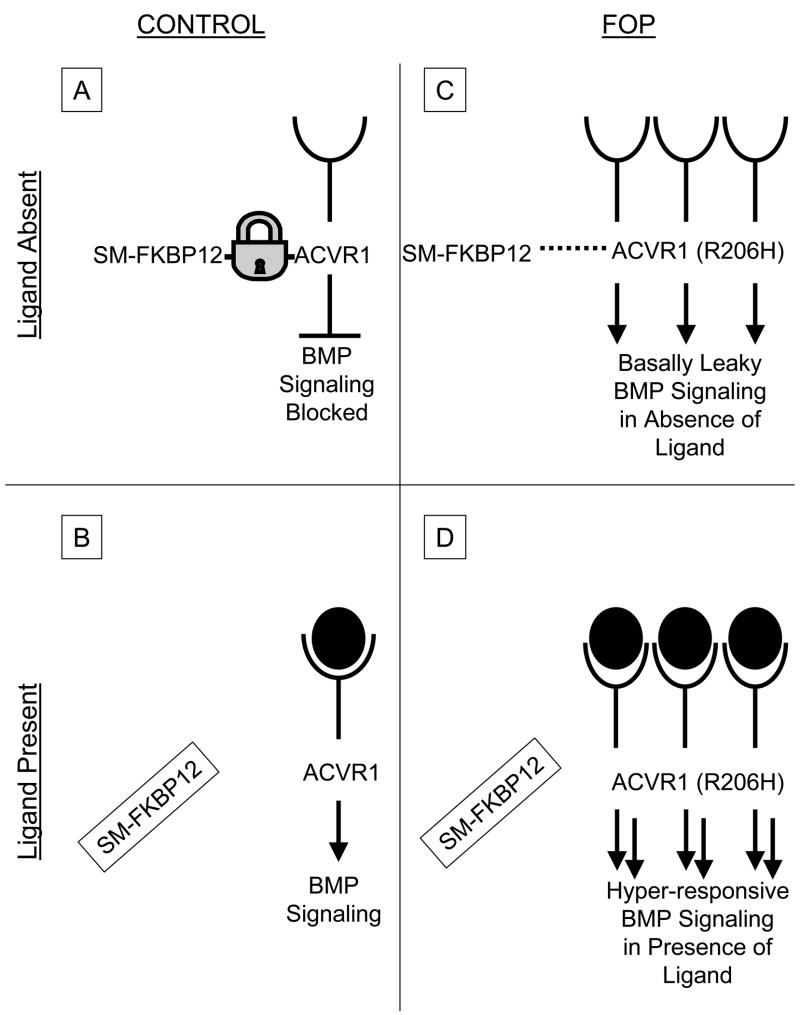
Figure 2.
Hypothetical schema of bone morphogenetic protein (BMP) signalling in fibrodysplasia ossificans progressiva (FOP) cells. In control cells (A), in the absence of ligand, the Smad/Smurf-FKBP12 (SM-FKBP12) complex binds activin receptor IA (ACVR1; a BMP type I receptor) and prevents its promiscuous phosphorylation by the constitutively active type II BMP receptor (not shown). SM-FKBP12 also promotes ubiquitin-associated degradation of ACVR1 in the absence of ligand, thus maintaining low steady-state levels of ACVR1 at the cell membrane. Following ligand binding in control cells (B), SM-FKBP12 dissociates from ACVR1, thus allowing the constitutively active BMP type II receptor (not shown) to phosphorylate ACVR1, and promote Smad 1, 5 and8 phosphorylation and downstream BMP signalling. In FOP cells, SM-FKBP12 does not appear to bind appropriately to the mutant receptor [ACVR1 (R206H)]. Thus, inhibition of BMP signalling is impaired in the absence of ligand, and basal leakiness of BMP signalling occurs (C). Additionally, it is suspected that since the SM-FKBP12 complex cannot properly target the mutant ACVR1 (R206H) receptor for ubiquitin-associated degradation, ACVR1 may be expected to accumulate at the cell surface. Thus, in the presence of ligand (D), hyper-responsive BMP signalling may be predicted to occur. Arrows, signalling promoted; blunt-end lines, signalling inhibited; lock, SM-FKBP12 binding to ACVR1; dashed lines, SM-FKBP12 binding to ACVR1 impaired; open cups, extracellular ligand-binding domain of ACVR1; filled-in circles, BMP ligand; filled-in circles inside open cups, BMP ligand binding to ACVR1.