Figure 7.
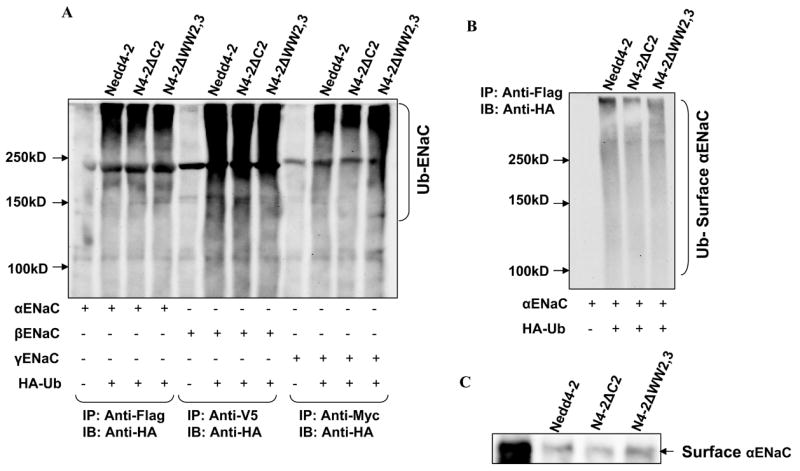
Effect of Nedd4-2 isoforms on ubiquitination of individual ENaC subunits: Panel A: Each of the Nedd4-2 isoforms were transfected with individual ENaC subunits and HA-tagged ubiquitin (HA-Ub) as indicated into HEK293 cells. ENaC subunits were immunoprecipitated with an anti-FLAG/V5/Myc antibody and immunoblotted with anti-HA to detect ubiquitinated α, β and γENaC respectively. Ubiquitinated proteins appear as high molecular mass smear. Each of the ENaC subunits when expressed alone can be ubiquitinated in the presence of each Nedd4-2 isoform. Panel B: αENaC was transfected with each of Nedd4-2 isoforms into HEK293 cells. Then biotinylated surface proteins were affinity purified and then immunoprecipitated with an anti-FLAG antibody and blotted with an anti-HA antibody. Each Nedd4-2 isoform ubiquitinates a surface expressed fraction of αENaC. Panel C: αENaC was transfected with each of Nedd4-2 isoforms into HEK293 cells and biotinylated surface proteins were blotted with an anti-FLAG antibody. Each of the Nedd4-2 isoforms reduces surface expression of αENaC correlating with ubiquitination seen in panel A and B.
