Fig. 2.
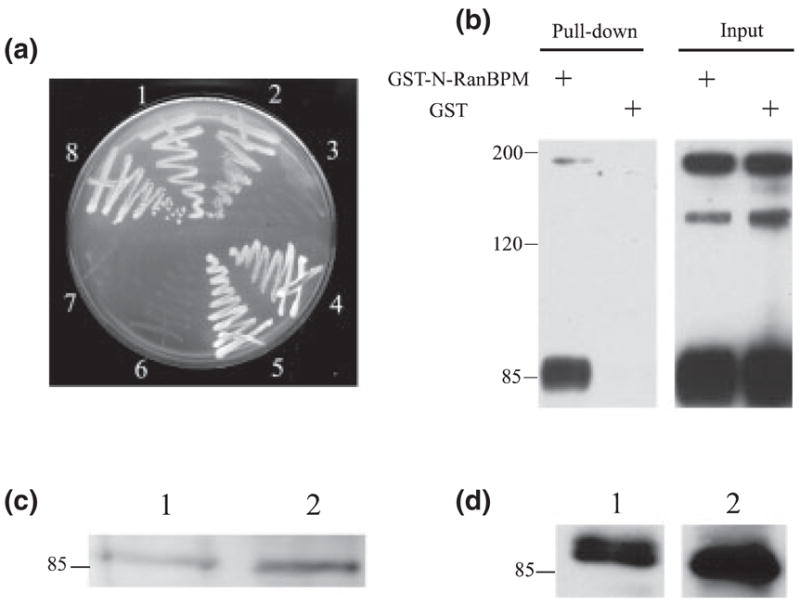
RanBPM interacts with L1 in vitro and in vivo, (a) Yeast AH109 cells were cotransformed and selected on a SD/-Trp/-Leu/-His/5 mM 3-aminotriazole plate. (1–3) AH109 yeast strain was cotransformed with pGBKT7-L1 cytoplasmic domain (L1CD)28 and the following GAL4 prey vectors: (1) pACT2-RanBPM; (2) pACT2-N-terminal region of RanBPM (N-RanBPM) and (3) pACT2-C-terminal region of RanBPM (C-RanBPM). (4–6) AH109 strain was cotransformed with pAS2-L1CD and the following GAL4 prey vectors: (4) pACT2-RanBPM; (5) pACT2-N-RanBPM and (6) pACT2-C-RanBPM. (7) Cotransformation of pGBKT7-L1CD28 and large T antigen-AD as negative control. (8) Cotransformation of p53-DBD and large T antigen-AD as positive control, (b) Glutathione S-transferase (GST)-N-RanBPM binds to purified L1 in GST pull-down assay. GST or GST-N-RanBPM was immobilized on beads and incubated with purified L1. L1 and an L1 fragment (85 kDa) containing the L1CD were detected on beads immobilized with GST-N-RanBPM but not on GST alone. (c and d) RanBPM was detected in L1 purified from brain. (1) Brain lysates as a positive control; (2) purified L1 from mouse brain. (c) Mouse anti-RanBPM; (d) rabbit anti-RanBPM.
