Abstract
Whether hematopoietic stem cells (HSCs) change with aging has been controversial. Previously, we showed that the HSC compartment in young mice consists of distinct subsets, each with predetermined self-renewal and differentiation behavior. Three classes of HSCs can be distinguished based on their differentiation programs: lymphoid biased, balanced, and myeloid biased. We now show that aging causes a marked shift in the representation of these HSC subsets. A clonal analysis of repopulating HSCs demonstrates that lymphoid-biased HSCs are lost and long-lived myeloid-biased HSCs accumulate in the aged. Myeloid-biased HSCs from young and aged sources behave similarly in all aspects tested. This indicates that aging does not change individual HSCs. Rather, aging changes the clonal composition of the HSC compartment. We show further that genetic factors contribute to the age-related changes of the HSC subsets. In comparison with B6 mice, aged D2 mice show a more pronounced shift toward myeloid-biased HSCs with a corresponding reduction in the number of both T- and B-cell precursors. This suggests that low levels of lymphocytes in the blood can be a marker for HSC aging. The loss of lymphoid-biased HSCs may contribute to the impaired immune response to infectious diseases and cancers in the aged.
Introduction
Traditionally, tissue stem cells were thought to be immortal and exempt from aging. Like all stem cells, hematopoietic stem cells (HSCs) self-renew, and serial transplantation studies show that populations of HSCs can live much longer than the donor from which they were originally isolated.1 HSCs express telomerase, necessary for chromosome stability during cell division.2 Together, these observations supported the idea of an immortal HSC. However, there is increasing evidence that HSCs isolated from aged donors are different from HSCs that are obtained from young donors.3,4 For example, aged HSCs may have a reduced ability to home to the bone marrow (BM),5,6 have an altered cell surface phenotype,5,7 may cycle more rapidly,5 produce more myeloid cells,5–8 and have a different gene expression program than their young counterparts.9,10 HSCs from aged environments may have slightly less self-renewal capacity than their younger counterparts.11 An accumulation of DNA damage in HSCs has been invoked to explain an age-related decline in HSCs.12,13
It should be emphasized that most of these age-related changes are controversial, and contradictory data have been published for almost every aspect of the behavior of aged HSCs. One of the reasons for these inconsistencies might be that most of the information about aged HSCs comes from comparisons of populations of HSCs from aged and young sources. However, it is clear by now that the HSC compartment consists of distinct subsets of HSCs.14–16 We showed that HSCs in these subsets are very different from each other, each possessing distinct self-renewal capacities, differentiation abilities, life span, and repopulation kinetics.14–16 Our work was later confirmed by others.17,18 Thus, if the representation of these HSC subsets changes during the life of an animal, population-based analysis of HSCs would not be informative.
We reasoned that a clonal analysis would interrogate the aging of the HSC compartment more precisely and might reconcile some of the contradictions in the field of HSC aging. For this analysis, we focused on 3 subsets of HSCs that we had identified previously in young mice.14,16,19 These subsets, which together make up the complete HSC compartment, are characterized by their ability to generate different levels of mature lymphoid and myeloid cells. The differentiation potential of balanced (Bala) HSCs is such that they generate myeloid and lymphoid cells in the same ratio as seen in the blood of unmanipulated mice. Myeloid-biased (My-bi) HSCs generate reduced numbers of lymphoid progeny, and lymphoid-biased (Ly-bi) HSCs generate few myeloid cells. The members of all 3 classes are true pluripotent HSCs in that they have self-renewal capacity and generate all cells of the hematopoietic lineages.
The myeloid bias of My-bi HSCs derives from an attenuated ability to generate developmentally early lymphoid precursors.14 Few prethymic T-cell precursors and B-cell precursors are made by My-bi HSCs. A blunted response of the lymphoid progeny to the central lymphokine IL-7 accounts, at least in part, for the diminished lymphopoiesis. My-bi HSCs from young mice show a delayed onset of repopulation following transplantation, but contribute significantly longer to peripheral hematopoiesis than other types of HSCs.14 A sluggish response to hematopoietic demand, a poor response to IL-7, and an attenuated production of lymphocytes also are characteristic for cells from aged animals.20–26 These similarities raised the possibility that the aged HSCs might be identical to My-bi HSCs.
To test this, we analyzed the clonal composition of aged HSCs from 2 strains of mice that are known to differ in HSC biology, C57BL/6 (B6) and DBA/2 (D2). B6 mice have low levels of HSCs throughout their life. In contrast, D2 mice have high levels of HSCs when young, but reportedly lose these in aging.3,6,27–31 Several genetic loci that contribute to a difference in HSC aging have been identified in mice28,31–34 and also in humans.35 Overall, there is evidence for a strong genetic component to the aging of the HSC. We created D2 mice congenic for the CD45 marker to permit a clonal analysis of D2 HSCs. Comparison of D2 and B6 HSCs shows for the first time that the clonal composition of the HSC compartment is controlled by both developmental and genetic differences. In both strains of mice, long-lived My-bi HSCs accumulated in the aged HSC compartment, albeit to a different extent. This presents a new mechanism for HSC aging where aging changes the HSC compartment but not the individual HSC.
Methods
Mice
D2 and B6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All other mice were bred at our facility. These included B6 mice transgenic for green fluorescent protein (B6-GFP), or B6 mice congenic for the CD45.1 allele (B6-CD45.1). D2-CD45.1Cms congenic mice were generated on the D2 background by backcrossing B6-CD45.1 to D2 mice for 11 generations. Thereafter, the D2-CD45.1 mice were maintained through sister-brother mating. B6-CD45.1, B6-GFP, and D2-CD45.1 mice served as donors in transplantation experiments. Breeder pairs of B6-W41W41 mice were generously provided by Dr David Harrison (The Jackson Laboratory); these mice have a mild mutation in ckit36 and served as hosts for some B6-based transplantation experiments.
Clonally repopulated mice
These were generated as described previously by in vivo limiting dilution approaches (LDAs).37 Donor cells were either unseparated BM cells or populations depleted of mature cells as lineagenegative (Lin−) cells by magnetic bead separation. We injected 2000 to 5000 unseparated BM cells containing limiting numbers (0.2-0.5) of HSCs.38,39 Lin− cells were approximately 10-fold enriched for HSCs and accordingly fewer cells were injected. Avidin-conjugated magnetic beads were purchased from B&D Biosciences (San Diego, CA). The lineage mix consisted of biotin-conjugated mAbs specific for B220, Gr-1, Mac-1, CD5, CD8, and the transferrin receptor. These were purified from culture supernatants of the respective hybridoma cells and conjugated in our laboratory. Hosts were generally lethally irradiated (2 doses of 5.5 Gy, 2 hours apart) D2 or B6 mice. A genetically distinguishable source of radioprotecting cells was coinjected. These were generally 2 × 105 twice-transplanted cells of host origin.40 Occasionally, we used sublethally irradiated (5 Gy) B6-W41W41 hosts for HSCs on the B6 background. There were no differences in HSC function in B6 and B6-W41/W41 hosts,37,41 and data were pooled from both systems. For serial transplantations, 5 × 106 BM cells from a primary host were injected into new, lethally irradiated hosts. For competitive repopulation experiments, lethally irradiated hosts were injected with equal numbers of test BM cells and young wild-type cells. Relative competitive repopulating units (rXRUs) were calculated based on the mean percentage donor-type cells, normalized by the number of cells injected as described.42,43 All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Sidney Kimmel Cancer Center (SKCC).
Determining lineage contribution
Mice were bled generally at 1, 3, 5, and 7 months after injection of HSCs. The fraction of donor-type cells in the lymphoid and myeloid lineages was measured by flow cytometry as described.19,37 Briefly, purified white blood cells from each mouse at each time point were split into 3 tubes, and cells in each tube were incubated with a mAb specific for the CD45.1 donor-type marker (or GFP expression was used as donor-type marker). At each time point, cells were also stained with Thy-1, B220, or Mac-1 and Gr-1 (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). These markers faithfully detect T or B lymphocytes or myeloid cells from both young and aged animals (Figure S2). Mice were considered to be repopulated if their blood contained 5% or more donor-type cells at least at 2 time points and if donor-type T and B lymphocytes, macrophages, and granulocytes were all present at the same time. HSCs were classified as Bala, My-bi, or Ly-bi in the primary recipient on the basis of the ratio of lymphocytes versus myeloid cells (L/M) in blood after donor-type cells had stabilized.14,16
IL-7 cultures
BM cells (1 × 106) were cultured in the presence of 10 ng IL-7 as described.14,44 Cells were obtained from clonally repopulated mice that showed at least 70% donor-type cells. Viable cells were enumerated after 7 days of cultures. Surviving cells were typed by immunofluorescence for expression of donor-type and lineage markers.
Colony-forming unit thymus assay
This is an in vivo limiting dilution assay that measures the ability of T-cell precursors to home directly to the thymus and proliferate in the thymic environment.45,46 Lethally irradiated B6 or D2 hosts were injected with 2 to 4 × 104 cells consisting of equal mixtures of congenic, genetically distinguishable BM from old and young donors. At the same time, each recipient received 106 host-type BM as competitor. Eleven to 20 mice were injected for each experiment. After 4 weeks, the percentage of donor-type cells in thymus derived from either the old or the young donors was determined by immunofluorescence. Background staining was in the order of 0.06% of the cells. The frequencies of colony-forming unit thymus (CFUt) assay were calculated based on the number of animals that did contain donor-type cells of either source.
Data evaluation
Significance values were calculated by Student t test unless otherwise stated. To approximate the output of differentiated cells per HSC clone during the first 7 months after transplantation, we used the area under the curve (AUC) as described.37 AUC was calculated with the Riemann-Lebesque sum approximation method (with % donor-type cells x as function values and t representing time in months). For LDA, the number of negative mice was used to calculate the frequency of HSCs or CFUt by maximum likelihood analysis.42,47
Results
Age- and strain-related changes in myeloid cells in blood
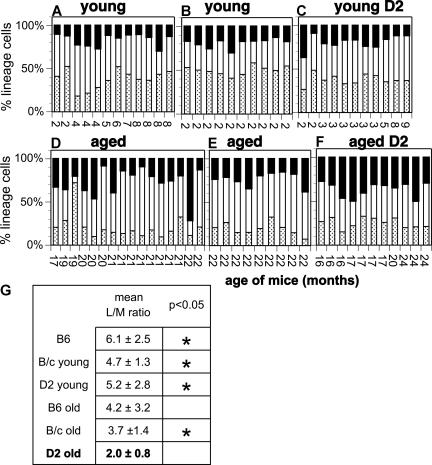
Human blood becomes increasingly biased toward myeloid cells in an age-related manner.48,49 Since mice have been used as model organisms to study human aging, it is of interest to determine whether they show a similar age-related increase in myeloid blood cells. To test this, we enumerated myeloid cells, and T and B lymphoid cells in the blood of normal young and aged B6, BALB/c (B/c), and D2 mice (Figure 1). We calculated the ratio of lymphoid to myeloid cells (L/M ratio) for each individual mouse and used these values to test for statistical significance. Young mice of all strains show lymphocyte-rich blood, and the L/M ratios of the 3 strains of mice do not differ (P = .36) from each other (Figure 1A-C,G). Aged B6 and B/c mice showed a slight but nonsignificant (P = .15) shift toward more myeloid cells in blood compared with young mice of the same strain (Figure 1D,E,G). Perhaps the most notable feature of the blood of aged B6 mice is the variability between individual mice with L/M ratios ranging from 0.6 to 11.9. The L/M ratios in the blood of aged D2 were more homogeneous (range, 1.0-3.4). In contrast to the other strains, but in analogy to humans, blood from aged D2 mice showed a significant (P = .001) increase in myeloid cells.
Figure 1.
Increased number of myeloid cells in the blood of aged D2. The percentage of myeloid cells (■), and B (□) and T ( ) lymphocytes in blood was measured from young (A-C) and aged (D-F) mice on the B6 (A,D), B/c (B,E), and D2 (C,F) background. Each bar represents the measurements from an individual mouse. The age of each mouse is indicated on the x-axis. Data are normalized to 100% to facilitate comparison. The mean L/M ratio and standard deviation for each group is shown in panel G. An * indicates that L/M ratios were significantly different from those obtained for aged D2 mice.
) lymphocytes in blood was measured from young (A-C) and aged (D-F) mice on the B6 (A,D), B/c (B,E), and D2 (C,F) background. Each bar represents the measurements from an individual mouse. The age of each mouse is indicated on the x-axis. Data are normalized to 100% to facilitate comparison. The mean L/M ratio and standard deviation for each group is shown in panel G. An * indicates that L/M ratios were significantly different from those obtained for aged D2 mice.
It has been reported that the rate of thymic involution is faster in D2 than in B6 mice.50,51 We found that aged mice of all strains showed a highly significant (P < .001) decline of T cells in blood compared with younger animals of the same strain (Figure 1). However, aged D2 mice showed a significantly (P = .02) higher level of T cells (25.4% ± 5.7%) compared with aged B6 (17.6% ± 6.7%). Thus, decreased T-cell generation alone cannot account for the myeloid bias of aged D2. Rather, a more generalized decline of lymphocytes is involved. This hinted at a change in the differentiation potential of a developmentally early cell, such as the HSC, in aging.
HSCs in D2 mice
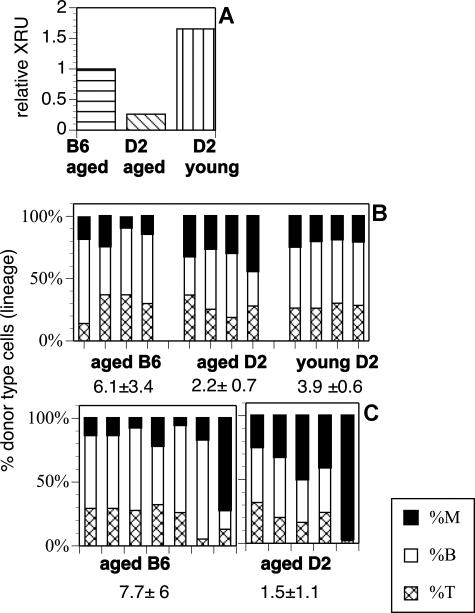
Most in vivo research on HSCs focuses on the B6 strain of mice because of the availability of convenient congenic mice on this background. Genetic markers, such as the allelic forms of the CD45 antigen, have been used extensively to distinguish donor- from host-type cells. To facilitate transplantation analyses for the strain D2, the CD45.1 allele was bred onto the D2 background by backcrossing B6-CD45.1 mice to D2 (CD45.2). Hematopoietic cells from these D2-CD45.1 mice express the CD45.1 antigen at levels comparable with those of the B6-CD45.1 strain (Figure S3). The numbers of HSCs in the parental and the congenic strain were not significantly different from each other when measured as long-term culture-initiating cells (P = .67 from 3 experiments). D2-CD45.1 and wild-type D2 BM cells, both from young mice, were competed against each other in vivo. The relative competitive repopulating units (rXRUs) of wild-type and congenic HSCs were not statistically different (P = .22) measured at 1, 3, 5, and 7 months (Figure 2A). A repeat experiment (currently at 3 months) confirms that D2 and D2-CD45.1 HSCs have similar repopulation capacity.
Figure 2.
Relative repopulation units and differentiation potential of HSCs from aged mice. (A) Relative competitive repopulating units (rXRUs) in aged D2 are lower than in young D2 or aged B6 mice. Four mice were injected with 5 × 105 BM cells in each group. All mice received an equal dose of competitor marrow. Horizontal stripes indicate aged B6-CD45.1 plus young B6; diagonal stripes, aged D2-CD45.1 plus young D2; and vertical stripes, young D2-CD45.1 plus young D2. Donor-type cells (CD45.1) in blood were enumerated 5 months later, and the number of rXRUs per 105 cells were calculated as described.42 (B) Lineage contribution of HSCs in competitive repopulation: Shown is the percentage of myeloid cells (■), B lymphocytes (□), and T lymphocytes (▩) of donor origin for each mouse in each group. The mice are from the experiment described in panel A. The mean L/M ratio (± SD) for each group is indicated. The difference between the L/M ratios of aged and young D2 (P = .008) is significant; the difference between aged D2 and aged B6 (P = .06) approaches significance. (C) Lineage in noncompetitive repopulation. Mice received 105 Lin− cells from aged B6 or D2 donors (noncompetitive). Each bar represents a different experiment with a different donor. The difference between the L/M ratios of D2 versus B6 mice is significant with P = .051.
The CD45-congenic D2 mouse allowed a direct comparison of HSC levels in young and aged mice. First, we measured the number of rXRUs (Figure 2A). BM cells from aged (20 months old) D2-CD45.1 mice were competed with BM cells from young (4 months) D2 mice. Aged D2 HSCs showed a distinct competitive disadvantage compared with young D2 HSCs. In contrast, aged and young B6 HSCs show similar levels of repopulation, resulting in an rXRU value of close to 1 (Figure 2A). Next, we measured the frequency of HSCs in (noncompetitive) limiting dilution analysis (Table 1). Clonally repopulated D2 and B6 hosts were generated by injecting freshly explanted BM cells (either unseparated or Lin−), containing limiting numbers (0.2-0.5) of HSCs, into ablated hosts. The results show that the frequency of clonally repopulating HSCs is 2- to 3-fold higher in D2 than in B6 mice. In agreement with previous reports,5,52,53 aged B6 have a slightly higher frequency of HSCs than young B6. Interestingly, the frequencies of clonally repopulating HSCs in aged and young D2 were not different. This suggests that the competitive repopulation capacity but not the number of HSCs is reduced in aged D2 mice.
Table 1.
Frequencies of HSCs in the BM of young and aged B6-CD45.1 and D2-CD45.1
| Cells injected | Unseparated BM |
Lin− |
||
|---|---|---|---|---|
| HSC frequency* | No.† | HSC frequency* | No.† | |
| D2 young | 24.7 (16.3-37.3) | 4 | 226.1 (136.7-373) | 3 |
| B6 young | 8.3 (5.4-12.7) | 5 | 100.6 (75-133.6) | 7 |
| D2 aged | ND | 248 (160-384.4) | 3 | |
| B6 aged | 11.3 (7.2-17.8) | 3 | 152.7 (77.6-300.4) | 1 |
Frequency of HSCs per 100 000 cells injected; the 95% confidence interval is given in parentheses.
No. indicates number of experiments. For each experiment 10 to 20 hosts were injected per cell dose. Data from experiments within a group were combined to calculate the frequency.
Next, we analyzed the distribution of myeloid and lymphoid cells among the donor-type cells in these experiments. Aged D2 HSCs gave rise to significantly higher levels of myeloid cells than either young D2 or aged B6 HSCs after transplantation. This was true whether cells were tested in competitive (Figure 2B) or noncompetitive, multiclonal (Figure 2C) repopulation assays. Thus, aged D2 HSCs recreated the myeloid bias seen in unmanipulated donors even after transplantation into young hosts and in the presence of young HSCs from the competitor graft. The data point to a HSC-intrinsic bias toward myeloid cell production with aging.
Age-related changes in the HSC compartment
As we showed previously, the lineage potential of individual HSCs is maintained through long-term, serial transplantation.14,16 Thus, the differentiation capacity is a stable, intrinsic property of HSCs. Yet, early after transplantation repopulation patterns are dynamic and there is an excess of myeloid cells regardless of the class of HSC injected (Figure S4). After 3 to 5 months, an equilibrium is reached and HSCs can be classified by the L/M ratio of the donor cells.14,16 My-bi HSCs generate L/M ratios smaller than 3 (but larger than 0), while Ly-bi HSCs generate L/M ratios greater than 10 (but smaller than infinity). Bala HSCs give rise to L/M ratios of 3 to 10. At all time points, all types of HSCs produce both myeloid and lymphoid cells as is expected from pluripotent HSCs (Figure S4).
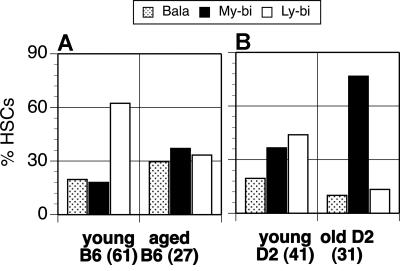
Using these criteria, we assessed the clonal composition of HSCs derived from young and aged D2 and B6 mice. An analysis of 61 individual HSC clones from young B6 mice showed that the HSC compartment is dominated by Ly-bi HSCs (Figure 3A). My-bi and Bala HSCs comprise 18% and 20% of all HSCs in young B6 mice. Compared with young B6, aged B6 mice had lost approximately half of the Ly-bi HSCs, and the levels of Bala and My-bi HSCs were increased 1.5- and 2-fold, respectively. The young D2 HSCs compartment also contains a majority of “youthful” Ly-bi, but young D2 have 2-fold more My-bi HSCs than young B6 (Figure 3B). Thus, young D2 and B6 differ in the composition of the HSC compartment, implying that genetic factors influence the clonal makeup of HSCs. This trend is enhanced with age. The aged D2 HSC compartment becomes dominated by My-bi HSCs, which now comprise 77% of all HSCs (Figure 3B). Ly-bi HSCs are reduced 3.3-fold and Bala HSCs by 2.2-fold in the aged D2 compartment. Thus, My-bi HSCs accumulate in the aged HSC compartment.
Figure 3.
Aging changes the composition of the HSC compartment. The percentage of HSC clones that had Bala ( ), My-bi (■), or Ly-bi (□) differentiation potential from young and aged animals is shown for B6 (A) and D2 (B). The number of individual HSC clones that was evaluated for each source of HSCs is indicated at the x-axis.
), My-bi (■), or Ly-bi (□) differentiation potential from young and aged animals is shown for B6 (A) and D2 (B). The number of individual HSC clones that was evaluated for each source of HSCs is indicated at the x-axis.
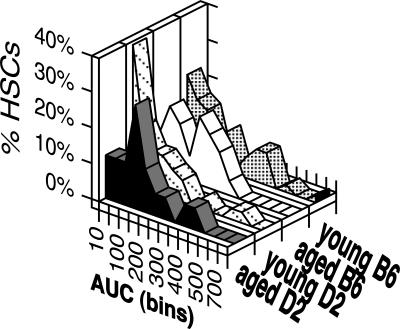
Next, we asked whether repopulation levels by individual HSCs differ in young and aged mice. The area under the curve (AUC) formed by the repopulation kinetics of individual HSCs in the first 7 months after transplantation can be used as an approximation of the output of differentiated cells by a clonally derived HSC.37 There were subtle changes in the distribution of aged and young HSCs (Figure 4) in that aged HSC compartments have lost the very largest HSC clones in both strains of mice. However, the median AUC values were not significantly different (P = .5), and all data sets showed very significant correlations (r2 > 0.88, P < .001). Similarly, when we focused the analysis on the AUC values generated by My-bi HSCs only, neither aged versus young HSCs nor HSCs from D2 versus B6 were significantly different from each other (P = .45). Thus, by this criterion, aging does not alter the output of differentiated progeny by individual HSCs.
Figure 4.
The repopulation capacity of HSC clones from young versus aged and in B6 versus D2 mice is similar. AUC values were calculated for each individual repopulation kinetic for the first 7 months after transplantation. These values were sorted into bins using increments of 50. Shown are the percentages of HSCs that fall into each of these bins for young B6 (46 clones), aged B6 (29), young D2 (34), and aged D2 (24) HSCs. This analysis includes only clones where 1, 3, 5, and 7 months of data were available. Not all clones analyzed in Figure 3 were analyzed at these time points.
Thymic precursors
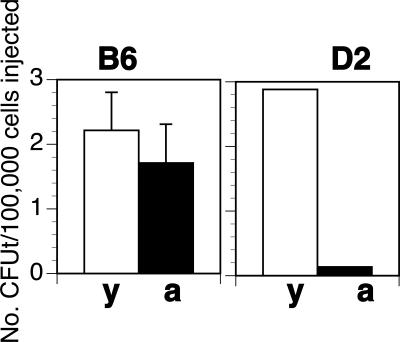
A dispute in the field of aging is whether T-cell precursors are reduced in aged animals.54 Since genetic differences control the composition of the HSC compartment, it was possible that the production of T-cell precursors in aging is controlled genetically. To test this, we used the colony-forming unit thymus (CFUt) assay that enumerates a BM-derived T-cell precursor that homes to the thymus where it proliferates and differentiates to form a colony of T-lineage cells.14,45 Levels of CFUt in aged and young B6 mice were not significantly (P = .3) different (Figure 5). In contrast, aged D2 showed 22-fold lower frequencies of CFUt than their young counterparts. Thus, D2 but not B6 mice show a decline in prethymic precursors. Moreover, the data support the interpretation that peripheral signs of aging (high myeloid count in blood) predict central age-related changes (loss of T-cell precursors).
Figure 5.
The number of colony-forming units thymus are reduced in aged D2 but not in B6 mice. Shown is the frequency of CFUt per 105 cells injected in B6 (left panel, mean and SD of 3 experiments) and D2 mice (right panel, 1 experiment using 20 hosts). Animals were injected with mixtures of young (y) and aged (a) BM with an excess of host-type BM. CFUt frequencies were calculated from the number of mice that had thymic donor-type cells derived from either the aged or the young donors at 4 weeks. For details, see “Colony-forming unit thymus assay.”
My-bi HSCs in aged D2
The data show a shift toward My-bi HSCs in aged mice of both strains. Are My-bi HSCs in young and aged animals the same type of HSC? The differentiation capacity of My-bi HSCs, like all HSCs, is fixed, and young My-bi HSCs will generate more My-bi HSCs upon self-renewal.16 To test whether aged My-bi HSCs behave in a similar fashion, 3 individual HSC clones were transplanted into multiple secondary recipients. All 3 My-bi HSCs gave rise to daughter HSCs that were also myeloid biased in their repopulation behavior (Figure S5). This indicates that aged and young My-bi HSCs share the stable imprinting of their differentiation capacity.
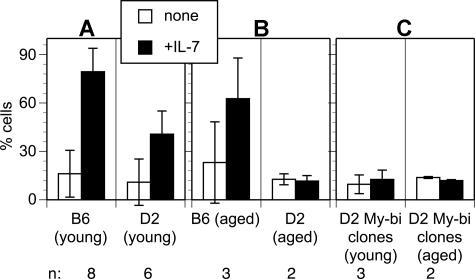
My-bi HSCs from young animals generate a normal-sized myeloid response but give rise to lymphoid progeny with a blunted response to the cytokine IL-7.14 To test whether My-bi HSCs from aged mice behave similarly, hosts were identified that had been clonally repopulated by My-bi HSCs originally isolated from aged D2 donors. BM cells were isolated, and the response of the progeny of these My-bi HSCs to IL-3 and IL-7 was tested. The number of colony-forming units culture (CFUc's) in response to IL-3 was not significantly (P = .3) different from that found in young D2 mice with 59.5 plus or minus 10.9 and 46.3 plus or minus 13.9 CFUc's (per 105 cells seeded) from unmanipulated and repopulated BM, respectively (2 experiments). Compared with the response of young pro-B cells (Figure 6A), the IL-7 response was diminished when cells from aged animals were tested (Figure 6B). Similarly, progeny from My-bi HSCs, derived originally from either young or aged D2 mice, showed a much reduced response to IL-7 (Figure 6C). Overall, the data indicate that My-bi HSCs from young and aged animals behave similarly to each other. An unexpected finding was that young D2 mice responded significantly less to IL-7 (P = .02) than B6 mice. This difference is even stronger in aged mice where there was a 6-fold difference in the recovery of B-lineage cells from D2 versus B6 after culture in IL-7. This suggests that the IL-7 response is controlled, at least in part, by genetic mechanisms.
Figure 6.
Blunted IL-7 response of pro-B cells derived from young and aged My-bi HSCs. BM cells from young (A) or aged (B) D2 or B6 mice were cultured in 10 ng/mL IL-7 for 1 week. BM cells from hosts, clonally repopulated by individual My-bi HSCs (C), were cultured in the same way. The data are expressed as mean percentage recovery of donor-type cells after culture (± SD). The number (n) of independent experiments performed is indicated at the bottom.
Clonal competition
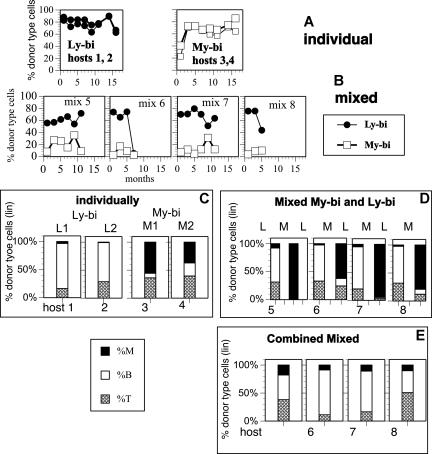
It was puzzling that HSCs from aged and young D2 did not differ in number and clonal output, but had a decreased competitive repopulation capacity (Figure 2). One possibility was that My-bi HSCs (which are highly enriched in aged D2) have a competitive disadvantage compared with other HSCs. To test this, a My-bi and a Ly-bi HSC clone (each from a young donor) were allowed to repopulate either alone (Figure 7A) or in competition with each other (Figure 7B) in secondary hosts. The My-bi and Ly-bi HSC clones had similar repopulation capacity over the 18 months that we followed them. However, when the clones were competed against each other, the Ly-bi HSC clone outcompeted the My-bi clone. Similar results were obtained in an independent experiment, where a My-bi clone was competed with a mixture of previously transplanted HSCs (data not shown). A possible reason for the advantage of the Ly-bi clone becomes apparent when the lineage contribution of the HSC clones is examined. The differentiation bias of each HSC clone is maintained. All daughter HSCs derived from the My-bi HSC are myeloid biased, and all daughters of the Ly-bi HSC are lymphoid biased (Figure 7C,D). However, in competition (Figure 7D), the myeloid bias of the My-bi HSC was enhanced to the point where very few lymphocytes were detected in most animals. This is consistent with the impaired fitness of My-bi HSC-derived lymphopoiesis. The data suggest that My-bi HSCs contribute little to lymphopoiesis when Ly-bi HSCs are present. Because lymphocytes comprise the vast majority of blood cells in young animals, My-bi HSCs appear to have a reduced repopulation capacity when competing with Ly-bi HSCs. When the lineage contributions of the 2 HSCs were combined (Figure 7E), overall repopulation (derived from both HSC clones) was balanced, demonstrating that detection of lineage bias is dependent on clonal repopulation.
Figure 7.
Clonal competition and obscuration. A My-bi (□) and a Ly-bi (●) HSC clone from young B6 donors were identified in primary hosts by their lineage contribution. BM cells (5 × 106) from the primary hosts were then injected into secondary hosts either alone (A,C) or as an equal mixture (B,D,E). Repopulation levels were measured at the indicated time points after transplantation into the secondary host. The HSC clones were originally obtained from a B6-GFP and a B6-CD45.1 donor, respectively, and the progenies of the clones were readily distinguishable. (A) Percentage donor-type cells in 2 mice each that received either the My-bi or the Ly-bi HSCs. (B) Percentage donor-type cells in 4 mice that received a 1:1 mixture of the My-bi and the Ly-bi HSCs. (C-E) The level of myeloid cells (■), B cells (□), and T cells (▩) in the donor-type cells at 7 months after transplantation except for mouse mix 8, where 5 months of data are presented. (C) Lineage contribution in mice that received either My-bi or Ly-bi HSCs. L1, L2, and M1 and M2 in panel C are the same mice shown in panel A. (D) Lineage contribution derived from the My-bi (M) and Ly-bi (L) HSCs in the mixed hosts. Each panel labeled host 5, 6, 7, and 8 corresponds to the mix 5, 6, 7, and 8, respectively, in panel B. Bars labeled L show the cells derived from the Ly-bi HSCs and bars labeled M show the cells derived from the My-bi HSCs in each host. (E) The percentage of myeloid and lymphoid cells derived from the 2 HSC clones shown in panel D was combined for each host.
Discussion
Human blood becomes markedly biased toward myeloid cells during adulthood and aging.48,49,55 Lymphocytes increase early in life, peaking at approximately 61% of white blood cells. Thereafter, the fraction of myeloid cells in blood increases and lymphocytes decline steadily until the age of 21 years, when a plateau is reached. Some studies indicate that lymphocyte counts decline further in the aged.49 Diminished lymphocyte production and function are major contributors to disease in the aged, and ways to alleviate age-related immune dysfunction are needed.
The age-related decrease in lymphocytes has been attributed to the decline of thymic function, but the loss of both B cells and T cells suggests a more general mechanism for age-related lymphopenia. The data that we present here support this view. We show here for the first time that aging is associated with a marked shift in the clonal composition of HSCs. In a dynamic process that involves both genetic and developmental factors, Ly-bi HSCs are lost and My-bi HSCs are enriched in aged mice. My-bi HSCs from young as well as aged donors are characterized by an attenuated ability to generate lymphocytes, a normal capacity for myelopoiesis, and a reduced competitive repopulation capacity. Thus, My-bi HSCs from both young and aged donors have many features previously attributed to aged HSCs. We propose that the major change caused by aging is a shift in the representation of the HSC classes rather than a change in the behavior of the HSCs.
The accumulation of My-bi HSCs in aging had a surprisingly large effect in the periphery. In aged D2 mice, the high level of My-bi HSCs translated into reduced levels of both B- and T-cell precursors and an increase in myeloid cells in blood. The data show for the first time that the composition of the HSC compartment influences the representation of mature cells in the periphery. This is surprising since mixtures of HSCs create an overall balanced repopulation pattern. Moreover, homeostatic proliferation in the periphery should compensate for the low lymphocyte production. On the other hand, there are thresholds below which peripheral homeostatic mechanisms cannot overcome a blunted generative potential.56 This suggests that the lymphoid output of the remaining Ly-bi and Bala HSCs cannot overcome the generative deficit of an HSC compartment that contains 77% My-bi HSCs in aged D2.
While the representation of My-bi HSCs is reflected in myeloid-biased blood in aged D2 mice, compensatory mechanisms must play a role in controlling levels of T and B lymphocytes in the blood. For example, aged D2 show high numbers of T cells in blood, even though thymus-homing T-cell precursors in the BM were strongly reduced. Aged B6 mice, on the other hand, have T-cell precursor levels that are similar to that found in young animals of the same strain. Yet, aged B6 have lower levels of T cells in blood than young B6 mice (P = .001). Indeed, each of the strains showed a signature age-related change in the composition of peripheral white blood cells. This supports the interpretation that genetic mechanisms contribute to the regulation of the peripheral levels of T and B cells in both young and aged mice. As an aside, the strong difference in prethymic precursor level in aged D2 versus B6 mice might account for the discrepancies in the literature about the effect of aging on T-cell precursors.
It is well known that D2 mice have a shorter life span than B6 mice,57 and the life span of mice has been correlated with HSC number and cycling.31,33 Our data now suggest that the level of lymphoid cells in blood predicts the aging of the HSC compartment, as reflected in the accumulation of My-bi HSCs. If an increase in myeloid cells in blood is taken as a marker of aging, then D2 mice age faster and/or at a more homogeneous pace than B6 mice. It is tempting to speculate that aging of stem cells affects the overall life span of the organism. Verification of this concept depends on testing whether other tissue stem cells show accelerated aging in D2 mice.
Our data show a strong genetic component to the age-related shift in the clonal representation of the HSC compartment. That HSCs in D2 and B6 mice age differently had been established in an elegant series of experiments that followed D2 and B6 HSCs in aggregation chimeras.58 In a subset of these chimeras, D2 HSCs stopped to contribute to peripheral hematopoiesis when mice aged. However, D2 HSCs were not lost and their presence was revealed when BM cells were transplanted into new hosts. This suggests that aged D2 HSCs are outcompeted by B6 HSCs in the aggregation chimeras. The reactivation of aged D2 HSCs in secondary hosts could be explained by a lack of competitive pressure early after transplantation, when HSCs of both genotypes struggle to fill up the hematopoietic compartments. In addition, early after transplantation all types of HSCs produce more myeloid than lymphoid cells. Overall, these data agree well with our observation that HSCs in aged D2 mice have the same number of HSCs, albeit with a diminished competitive repopulation capacity compared with young D2 HSCs.
Perhaps the simplest explanation for the accumulation of My-bi HSCs in the aged is that their long life span allows them to outlast both Ly-bi and Bala HSCs, which on average have shorter life spans.14 We cannot entirely exclude that the aged environment instructs other types of HSCs to switch to a myeloid-biased phenotype. However, young animals contain a sizable number of My-bi HSCs, which demonstrates that the aged environment is not necessary for the generation of My-bi HSCs. Despite the similarity of aged and young My-bi HSCs, age-related changes of HSCs cannot be ruled out entirely. It is, however, likely that such changes are subtle. Generally, aged HSCs show differences to young HSCs only when the cells are exposed to severe stress.12,13,59
An involvement of HSCs in an age-related myeloid shift has been previously surmised. Myeloid-dominant repopulation patterns were observed after transfer of Lin−Sca-1+ckit−CD34+ HSCs from aged B6.7 Because Lin−Sca-1+ckit−CD34+ from young mice did not show this behavior, it was concluded that the pathology of aging changes the function of all HSCs so that they produce more myeloid than lymphoid cells. However, this interpretation is problematic for several reasons. First, HSCs enriched by other methods from the same aged strain of mice did not generate myeloid-biased repopulation patterns.5 This is consistent with our data in as far as multiclonal populations of HSCs from aged B6 gave rise to balanced lineage compositions. Second, it is very clear that My-bi HSCs are found in young mice.14,60 Thus, myeloid bias is hardly unique to the aged. It is likely that the results presented by Sudo et al7 reflect a change in cell surface phenotype of young versus aged HSCs. The CD34− cell surface marker is well known to be changeably expressed on HSCs.61,62 The unseparated or minimally enriched Lin− HSCs used here avoided bias introduced by changeable phenotypes.
It is tempting to speculate that the same classes of HSCs exist in humans. Based on the number of myeloid cells in blood, one could assume that the more rapidly and vigorously contributing HSCs (Ly-bi and most Bala HSCs) exhaust during the first few years in large animals. These HSCs may be needed to seed the rapidly expanding lymphoid organs early in life. Thereafter, hematopoiesis in large animals may be derived from My-bi and a subset of balanced HSCs, which persist in the HSC compartment due to their long life span. This interpretation is supported by the observation that HSC-derived lymphocytic leukemia is found mostly in the young, while the incidence of myeloid leukemia increases with age. The loss of rapidly responding Ly-bi and Bala HSCs with their vigorous lymphocyte differentiation capacity may well contribute to the impaired immune response to infectious diseases and cancers in the aged. So far, attempts to bolster the immune response of the aged have met with very limited success. Perhaps therapy directed at HSCs will be more successful.
Supplementary Material
Acknowledgments
We thank Drs Joy Phillips (SKCC) and Becky Adkins (University of Miami, Miami, FL) for helpful discussions and editorial comments. The technical help of Michael McGary is acknowledged.
This work was supported by National Institutes of Health (Bethesda, MD) grants AG023197 and DK048015.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: R.H.C. and C.E.M.-S. designed and performed experiments; C.E.M.-S. and H.B.S. evaluated the data; C.E.M.-S. designed the project and wrote the paper; all authors read and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christa E. Muller-Sieburg, Sidney Kimmel Cancer Center, 10905 Road to the Cure, San Diego, CA 92121; e-mail: cmuller@skcc.org.
References
- 1.Harrison DE. Normal production of erythrocytes by mouse marrow continuous for 73 months. Proc Natl Acad Sci U S A. 1973;70:3184–3188. doi: 10.1073/pnas.70.11.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yui J, Chiu CP, Lansdorp PM. Telomerase activity in candidate stem cells from fetal liver and adult bone marrow. Blood. 1998;91:3255–3262. [PubMed] [Google Scholar]
- 3.Geiger H, Van Zant G. The aging of lympho-hematopoietic stem cells. Nat Immunol. 2002;3:329–333. doi: 10.1038/ni0402-329. [DOI] [PubMed] [Google Scholar]
- 4.Marley SB, Lewis JL, Davidson RJ, et al. Evidence for a continuous decline in haemopoietic cell function from birth: application to evaluating bone marrow failure in children. Br J Haematol. 1999;106:162–166. doi: 10.1046/j.1365-2141.1999.01477.x. [DOI] [PubMed] [Google Scholar]
- 5.Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nat Med. 1996;2:1011–1016. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- 6.Liang Y, Van Zant G, Szilvassy SJ. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood. 2005;106:1479–1487. doi: 10.1182/blood-2004-11-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sudo K, Ema H, Morita Y, Nakauchi H. Age-associated characteristics of murine hematopoietic stem cells. J Exp Med. 2000;192:1273–1280. doi: 10.1084/jem.192.9.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim M, Moon HB, Spangrude GJ. Major age-related changes of mouse hematopoietic stem/progenitor cells. Ann N Y Acad Sci. 2003;996:195–208. doi: 10.1111/j.1749-6632.2003.tb03247.x. [DOI] [PubMed] [Google Scholar]
- 9.Rossi DJ, Bryder D, Zahn JM, et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A. 2005;102:9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, Goodell MA. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5:1750–1762. doi: 10.1371/journal.pbio.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamminga LM, van Os R, Ausema A, et al. Impaired hematopoietic stem cell functioning after serial transplantation and during normal aging. Stem Cells. 2005;23:82–92. doi: 10.1634/stemcells.2004-0066. [DOI] [PubMed] [Google Scholar]
- 12.Nijnik A, Woodbine L, Marchetti C, et al. DNA repair is limiting for haematopoietic stem cells during ageing. Nature. 2007;447:686–690. doi: 10.1038/nature05875. [DOI] [PubMed] [Google Scholar]
- 13.Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- 14.Muller-Sieburg CE, Cho RH, Karlsson L, Huang J-F, Sieburg HB. Myeloid-biased hematopoietic stem cells have extensive self-renewal capacity but generate diminished lymphoid progeny with impaired IL-7 responsiveness. Blood. 2004;103:4111–4118. doi: 10.1182/blood-2003-10-3448. [DOI] [PubMed] [Google Scholar]
- 15.Muller-Sieburg CE, Sieburg HB. Clonal diversity of the stem cell compartment. Curr Opin Hematol. 2006;13:243–248. doi: 10.1097/01.moh.0000231421.00407.65. [DOI] [PubMed] [Google Scholar]
- 16.Muller-Sieburg CE, Cho RH, Thoman M, Adkins B, Sieburg HB. Deterministic regulation of hematopoietic stem cell self-renewal and differentiation. Blood. 2002;100:1302–1309. [PubMed] [Google Scholar]
- 17.Dykstra B, Kent D, Bowie M, et al. Long-term propagation of distinct hematopoietic differentiation programs in vivo. Cell Stem Cell. 2007;1:218–229. doi: 10.1016/j.stem.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 18.Graf T. Two lineages of hematopoietic stem cells identified by differences in lineage priming. [Abstract.] Exp Hematol. 2007;35(suppl 1) [Google Scholar]
- 19.Cho RH, Muller-Sieburg CE. High frequency of long-term culture-initiating cells retain in vivo repopulation and self-renewal capacity. Exp Hematol. 2000;28:1080–1086. doi: 10.1016/s0301-472x(00)00507-5. [DOI] [PubMed] [Google Scholar]
- 20.Stephan RP, Lill-Elghanian DA, Witte PL. Development of B cells in aged mice: decline in the ability of pro-B cells to respond to IL-7 but not to other growth factors. J Immunol. 1997;158:1598–1609. [PubMed] [Google Scholar]
- 21.Miller JP, Allman D. The decline in B lymphopoiesis in aged mice reflects loss of very early B-lineage precursors. J Immunol. 2003;171:2326–2330. doi: 10.4049/jimmunol.171.5.2326. [DOI] [PubMed] [Google Scholar]
- 22.Andrew D, Aspinall R. IL-7 and not stem cell factor reverses both the increase in apoptosis and the decline in thymopoiesis seen in aged mice. J Immunol. 2001;166:1524–1530. doi: 10.4049/jimmunol.166.3.1524. [DOI] [PubMed] [Google Scholar]
- 23.Eren R, Globerson A, Abel L, Zharhary D. Quantitative analysis of bone marrow thymic progenitors in young and aged mice. Cell Immunol. 1990;127:238–246. doi: 10.1016/0008-8749(90)90129-f. [DOI] [PubMed] [Google Scholar]
- 24.Tyan ML. Impaired thymic regeneration in lethally irradiated mice given bone marrow from aged donors. Proc Soc Exp Biol Med. 1976;152:33–35. doi: 10.3181/00379727-152-39321. [DOI] [PubMed] [Google Scholar]
- 25.Thoman ML. Early steps in T cell development are affected by aging. Cell Immunol. 1997;178:117–123. doi: 10.1006/cimm.1997.1133. [DOI] [PubMed] [Google Scholar]
- 26.Miller JP, Allman D. Linking age-related defects in B lymphopoiesis to the aging of hematopoietic stem cells. Semin Immunol. 2005;17:321–329. doi: 10.1016/j.smim.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Muller-Sieburg CE, Riblet R. Genetic control of the frequency of hematopoietic stem cells in mice: mapping of a candidate locus to chromosome 1. J Exp Med. 1996;183:1141–1150. doi: 10.1084/jem.183.3.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J, Astle CM, Harrison DE. Genetic regulation of primitive hematopoietic stem cell senescence. Exp Hematol. 2000;28:442–450. doi: 10.1016/s0301-472x(99)00157-5. [DOI] [PubMed] [Google Scholar]
- 29.Liang Y, Jansen M, Aronow B, Geiger H, Van Zant G. The quantitative trait gene latexin influences the size of the hematopoietic stem cell population in mice. Nat Genet. 2007;39:178–188. doi: 10.1038/ng1938. [DOI] [PubMed] [Google Scholar]
- 30.de Haan GVZG. Intrinsic and extrinsic control of hemopoietic stem cell numbers: mapping of a stem cell gene. J Exp Med. 1997;186:529–536. doi: 10.1084/jem.186.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Haan GNW, Van Zant G. Mouse strain-dependent changes in frequency and proliferation of hematopoietic stem cells during aging: correlation between lifespan and cycling activity. Blood. 1997;89:1543–1550. [PubMed] [Google Scholar]
- 32.Geiger H, True JM, de Haan G, Van Zant G. Age- and stage-specific regulation patterns in the hematopoietic stem cell hierarchy. Blood. 2001;98:2966–2972. doi: 10.1182/blood.v98.10.2966. [DOI] [PubMed] [Google Scholar]
- 33.Henckaerts E, Langer JC, Snoeck HW. Quantitative genetic variation in the hematopoietic stem cell and progenitor cell compartment and in lifespan are closely linked at multiple loci in BXD recombinant inbred mice. Blood. 2004;104:374–379. doi: 10.1182/blood-2003-12-4304. [DOI] [PubMed] [Google Scholar]
- 34.Geiger H, Rennebeck G, Van Zant G. Regulation of hematopoietic stem cell aging in vivo by a distinct genetic element. Proc Natl Acad Sci U S A. 2005;102:5102–5107. doi: 10.1073/pnas.0408654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abkowitz JL, Taboada M, Shelton GH, Catlin SN, Guttorp P, Kiklevich JV. An X chromosome gene regulates hematopoietic stem cell kinetics. Proc Natl Acad Sci U S A. 1998;95:3862–3866. doi: 10.1073/pnas.95.7.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harrison DE, Astle CM. Lymphoid and erythroid repopulation in B6 W-anemic mice: a new unirradiated recipient. Exp Hematol. 1991;19:374–377. [PubMed] [Google Scholar]
- 37.Sieburg HB, Cho RH, Dykstra B, Uchida N, Eaves CJ, Muller-Sieburg CE. The hematopoietic stem compartment consists of a limited number of discrete stem cell subsets. Blood. 2006;107:2311–2316. doi: 10.1182/blood-2005-07-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakano T, Waki N, Asai H, Kitamura Y. Effect of 5-fluorouracil on “primitive” hematopoietic stem cells that reconstitute whole erythropoiesis of genetically anemic W/Wv mice. Blood. 1989;73:425–430. [PubMed] [Google Scholar]
- 39.Trevisan M, Yan XQ, Iscove NN. Cycle initiation and colony formation in culture by murine marrow cells with long-term reconstituting potential in vivo. Blood. 1996;88:4149–4158. [PubMed] [Google Scholar]
- 40.Szilvassy SJ, Humphries RK, Lansdorp PM, Eaves AC, Eaves CJ. Quantitative assay for totipotent reconstituting hematopoietic stem cells by a competitive repopulation strategy. Proc Natl Acad Sci U S A. 1990;87:8736–8740. doi: 10.1073/pnas.87.22.8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uchida N, Dykstra B, Lyons KJ, Leung FY, Eaves CJ. Different in vivo repopulating activities of purified hematopoietic stem cells before and after being stimulated to divide in vitro with the same kinetics. Exp Hematol. 2003;12:1338–1347. doi: 10.1016/j.exphem.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Wineman JP, Nishikawa S, Muller-Sieburg CE. Maintenance of high levels of pluripotent hematopoietic stem cells in vitro: effect of stromal cells and c-kit. Blood. 1993;81:365–372. [PubMed] [Google Scholar]
- 43.Harrison D, Jordan C, Zhong R, Astle C. Primitive hemopoietic stem cells: direct assay of most productive populations by competitive repopulation with simple bionomial, correlation and covariance calculation. Exp Hematol. 1993;21:206–219. [PubMed] [Google Scholar]
- 44.Sherwood EM, Xu W, Riley RL. B cell precursors in senescent mice exhibit decreased recruitment into proliferative compartments and altered expression of Bcl-2 family members. Mech Ageing Dev. 2003;124:147–153. doi: 10.1016/s0047-6374(02)00042-8. [DOI] [PubMed] [Google Scholar]
- 45.Ezine S, Weissman IL, Rouse RV. Bone marrow cells give rise to distinct cell clones within the thymus. Nature. 1984;309:629–631. doi: 10.1038/309629a0. [DOI] [PubMed] [Google Scholar]
- 46.Spangrude GJ, Muller-Sieburg CE, Heimfeld S, Weissman IL. Two rare populations of mouse Thy-1lo bone marrow cells repopulate the thymus. J Exp Med. 1988;167:1671–1683. doi: 10.1084/jem.167.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhong RK, Astle CM, Harrison DE. Distinct developmental patterns of short-term and long-term functioning lymphoid and myeloid precursors defined by competitive limiting dilution analysis in vivo. J Immunol. 1996;157:138–145. [PubMed] [Google Scholar]
- 48.Ryan DE. Examination of the Blood. 6th ed. New York, NY: McGrawHill Book; 2001. [Google Scholar]
- 49.Lichtman MA, Williams WJ. Hamatology in the Aged. 6th ed. New York, NY: McGrawHill Book; 2001. [Google Scholar]
- 50.Hsu HC, Mountz JD, Williams RW, et al. Age-related change in thymic T-cell development is associated with genetic loci on mouse chromosomes 1, 3, and 11. Mech Ageing Dev. 2002;123:1145–1158. doi: 10.1016/s0047-6374(02)00004-0. [DOI] [PubMed] [Google Scholar]
- 51.Mountz JD, Van Zant GE, Zhang HG, et al. Genetic dissection of age-related changes of immune function in mice. Scand J Immunol. 2001;54:10–20. doi: 10.1046/j.1365-3083.2001.00943.x. [DOI] [PubMed] [Google Scholar]
- 52.Harrison DE, Astle CM, Stone M. Numbers and functions of transplantable primitive immunohematopoietic stem cells: effects of age. J Immunol. 1989;142:3833–3840. [PubMed] [Google Scholar]
- 53.Yilmaz OH, Kiel MJ, Morrison SJ. SLAM family markers are conserved among hematopoietic stem cells from old and reconstituted mice and markedly increase their purity. Blood. 2006;107:924–930. doi: 10.1182/blood-2005-05-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Linton P, Thoman ML. T cell senescence. Front Biosci. 2001;6:D248–D261. doi: 10.2741/linton. [DOI] [PubMed] [Google Scholar]
- 55.Rothstein G. Hematopoiesis in the aged: a model of hematopoietic dysregulation? Blood. 1993;82:2601–2604. [PubMed] [Google Scholar]
- 56.Agenes F, Rosado MM, Freitas AA. Independent homeostatic regulation of B cell compartments. Eur J Immunol. 1997;27:1801–1807. doi: 10.1002/eji.1830270731. [DOI] [PubMed] [Google Scholar]
- 57.Klebanov S, Astle CM, Roderick TH, et al. Maximum life spans in mice are extended by wild strain alleles. Exp Biol Med (Maywood) 2001;226:854–859. doi: 10.1177/153537020122600908. [DOI] [PubMed] [Google Scholar]
- 58.Van Zant G, Holland BP, Eldridge PW, Chen JJ. Genotype-restricted growth and aging patterns in hematopoietic stem cell populations of allophenic mice. J Exp Med. 1990;171:1547–1565. doi: 10.1084/jem.171.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Janzen V, Forkert R, Fleming HE, et al. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- 60.Jordan CT, Lemischka IR. Clonal and systemic analysis of long-term hematopoiesis in the mouse. Genes Dev. 1990;4:220–232. doi: 10.1101/gad.4.2.220. [DOI] [PubMed] [Google Scholar]
- 61.Sato T, Laver JH, Ogawa M. Reversible expression of CD34 by murine hematopoietic stem cells. Blood. 1999;94:2548–2554. [PubMed] [Google Scholar]
- 62.Camargo FD, Chambers SM, Drew E, McNagny KM, Goodell MA. Hematopoietic stem cells do not engraft with absolute efficiencies. Blood. 2006;107:501–507. doi: 10.1182/blood-2005-02-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.