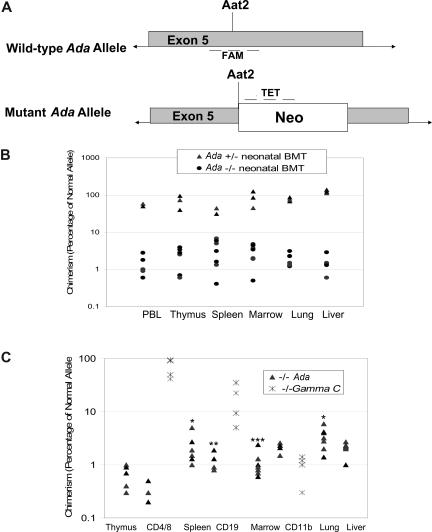
Figure 4.
Determination of donor chimerism after neonatal BMT. (A) Schematic representation of qPCR approach for determining donor chimerism. A real-time quantitative PCR (qPCR) primer/probe set was designed to amplify the normal, wild-type Ada allele at the site of disruption in the mutant allele by insertion of the Neo gene at the unique Aat2 site in exon 5. A second set of primers/probe was designed to the neomycin resistance gene inserted at the Aat2 site in the mutant allele. (B) Chimerism at 16 days after neonatal BMT. Whole litters borne of heterozygous matings were injected with normal donor bone marrow within the first 1 to 3 days after birth; the individual genotypes were subsequently determined from tail DNA. Mice were killed at 16 days of age, and DNA from tissues was analyzed to measure donor chimerism. Both homozygous Ada−/− mice and heterozygous +/− mice were analyzed, with the heterozygote littermates (50% normal allele) serving as internal controls for the qPCR measurements. (C) Chimerism at 60 days after neonatal BMT. In ADA-deficient mice (n = 7), chimerism was determined at day 60 after neonatal BMT, analyzing DNA from tissue fragments as well as from the indicated cell subpopulations isolated with immunomagnetic beads from thymus (CD4+ and CD8+ T cells), spleen (CD19+ B cells), and bone marrow (CD11b+ myeloid cells). γC gene knockout mice (Ly5.1) were treated by neonatal infusion of normal congenic bone marrow (Ly5.2, γC+/+) and killed after 60 days (n = 4). Cells from thymus (CD4+ and CD8+ T cells), spleen (CD19+ B cells), and bone marrow (CD11b+ myeloid cells) were analyzed by flow cytometry to measure donor chimerism, based on the percentage of cells of the indicated cell subpopulations expression Ly5.2. Statistical analysis for Ada−/− only: *Significantly higher than thymus (P < .05), marrow (P < .05), and liver (P < .05). **Significantly higher than CD4 (P < .01). ***Significantly higher than CD19 (P < .006) and CD4 (P < .001).