Figure 2. Tunnels from the protein surface to the active site.
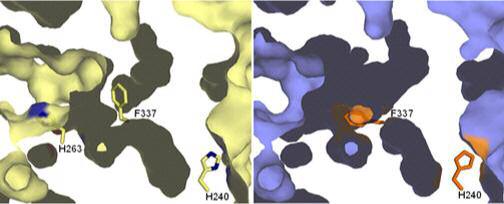
A cross section through the active site of the wild-type and the H263C variant human ferrochelatases is shown. The active site opening is to the left and back. The wild-type surface is colored in cream and the H263C variant surface is in slate. The position of the centrally located and essential H263 is shown in the wild-type structure in cream and blue for carbon and nitrogen, respectively. The figure illustrates that the reorientation of the residues described in the text which result in the closing off of the tunnel opening seen at the bottom of this diagram and the opening of another tunnel entrance at the top of the picture. These alterations result mainly from the movement of the side chain of F337, shown as sticks in the middle of the diagram. The expansion of the pocket seen at the top of the diagram is attributable to the reorientation of the side chain of R164.
