Scheme 3.
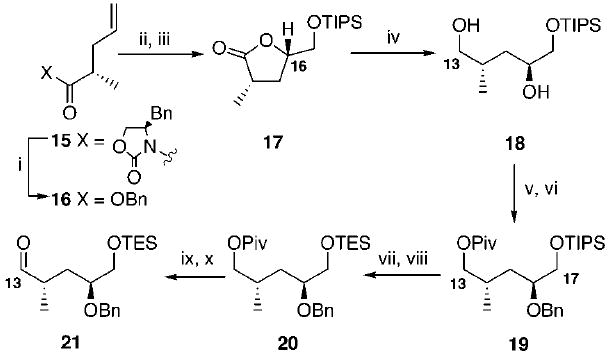
Synthesis of the C16 Benzyloxy-Substituted Aldehydea
a Key: (i) BnOLi, PMBOH, THF, 99%; (ii) AD mix α, NaHCO3, t-BuOH, H2O; (iii) TIPSOTf, 2,6-lutidine, CH2Cl2, −78 °C, 66% overall yield from 16 (3:1 d.s.); (iv) LiBH4, MeOH, THF, 0 °C, 99%; (v) PivCl, Et3N, DMAP, CH2Cl2, 66%; (vi) NaH, BnBr, DMF, −50 °C to −10 °C; (vii) TBAF, THF, 79% (over two steps); (viii) TESCl, DMAP, Et3N, CH2Cl2, 95%; (ix) LiBH4, saturated aqueous NH4Cl, THF, 0 °C to rt, 99%; (x) TPAP, NMO, CH2Cl2, molecular sieves, 87%.
