Abstract
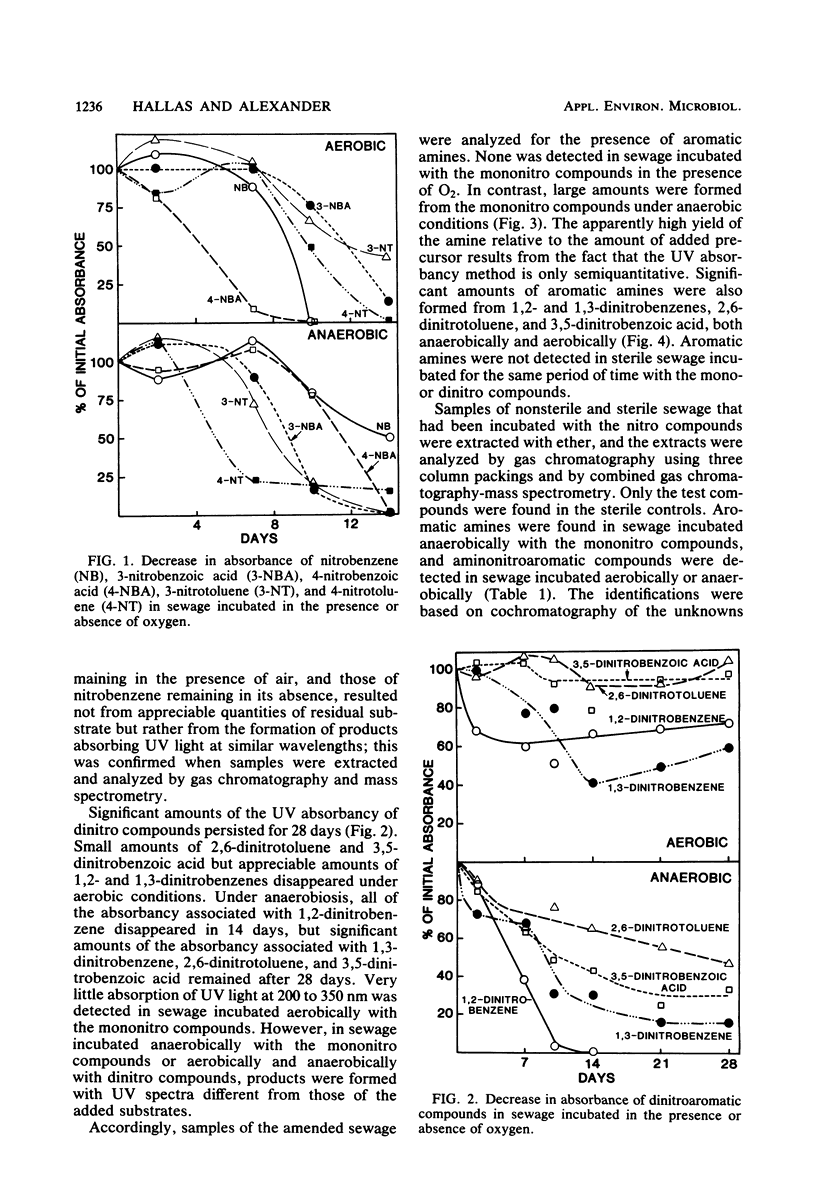
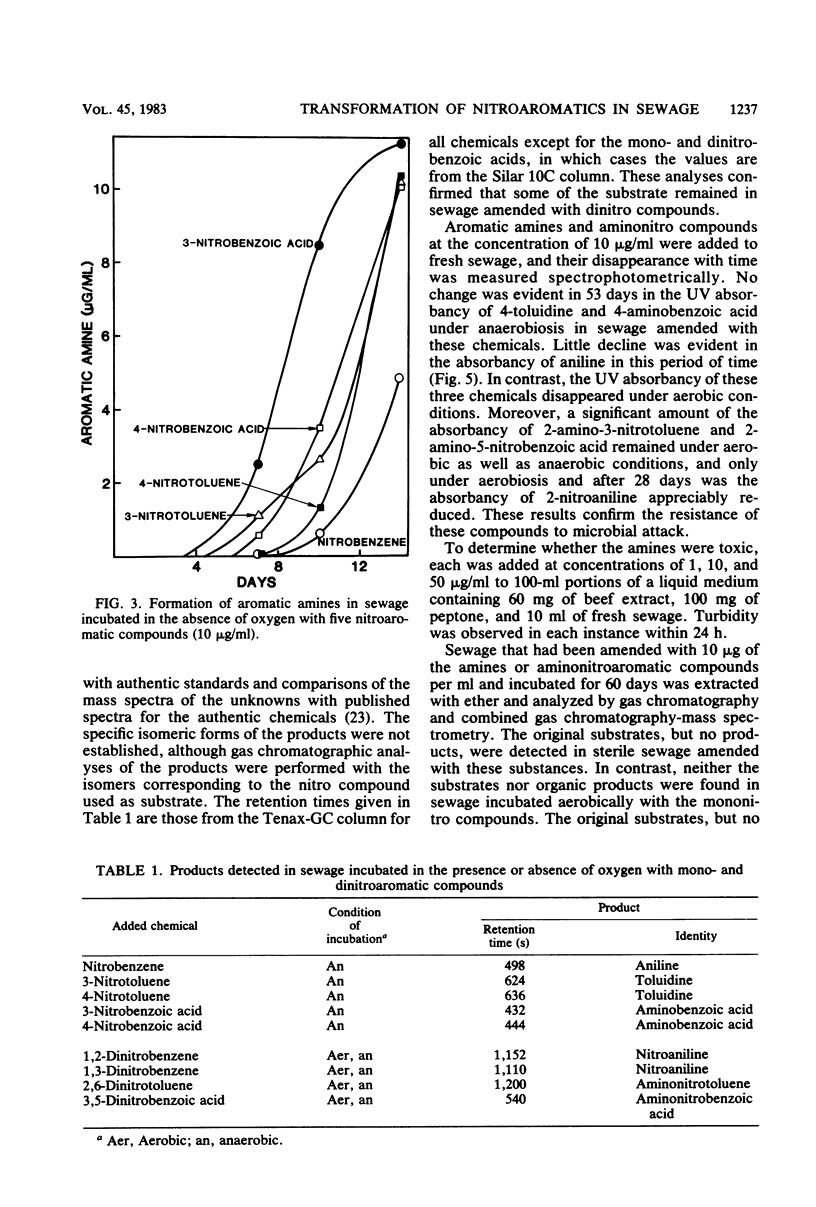
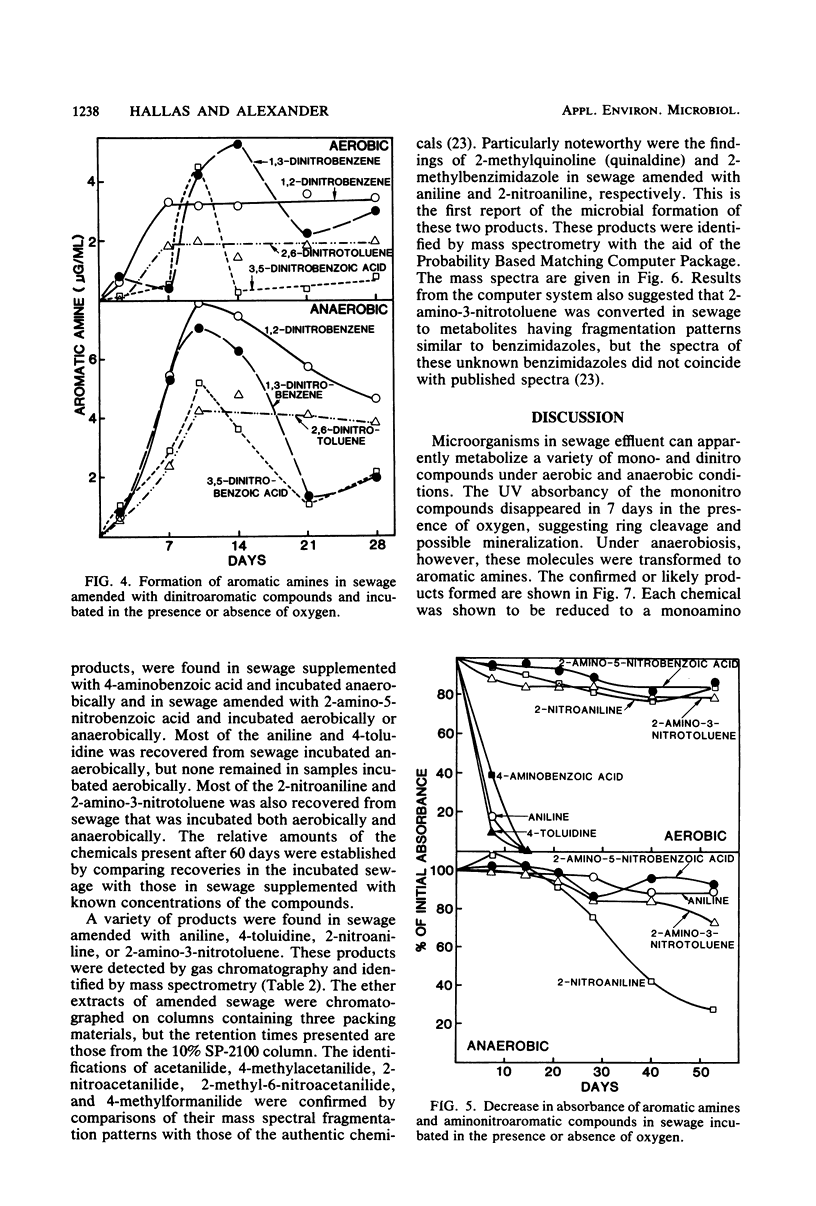
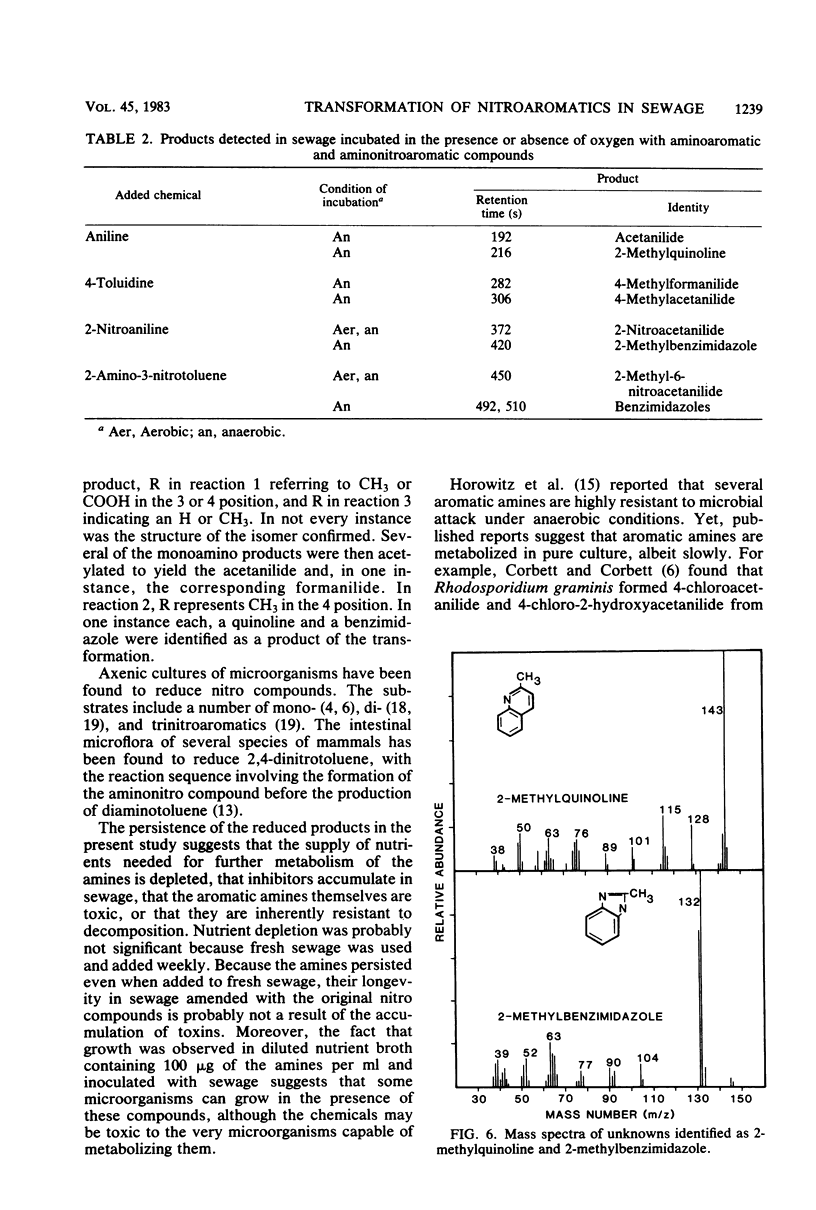
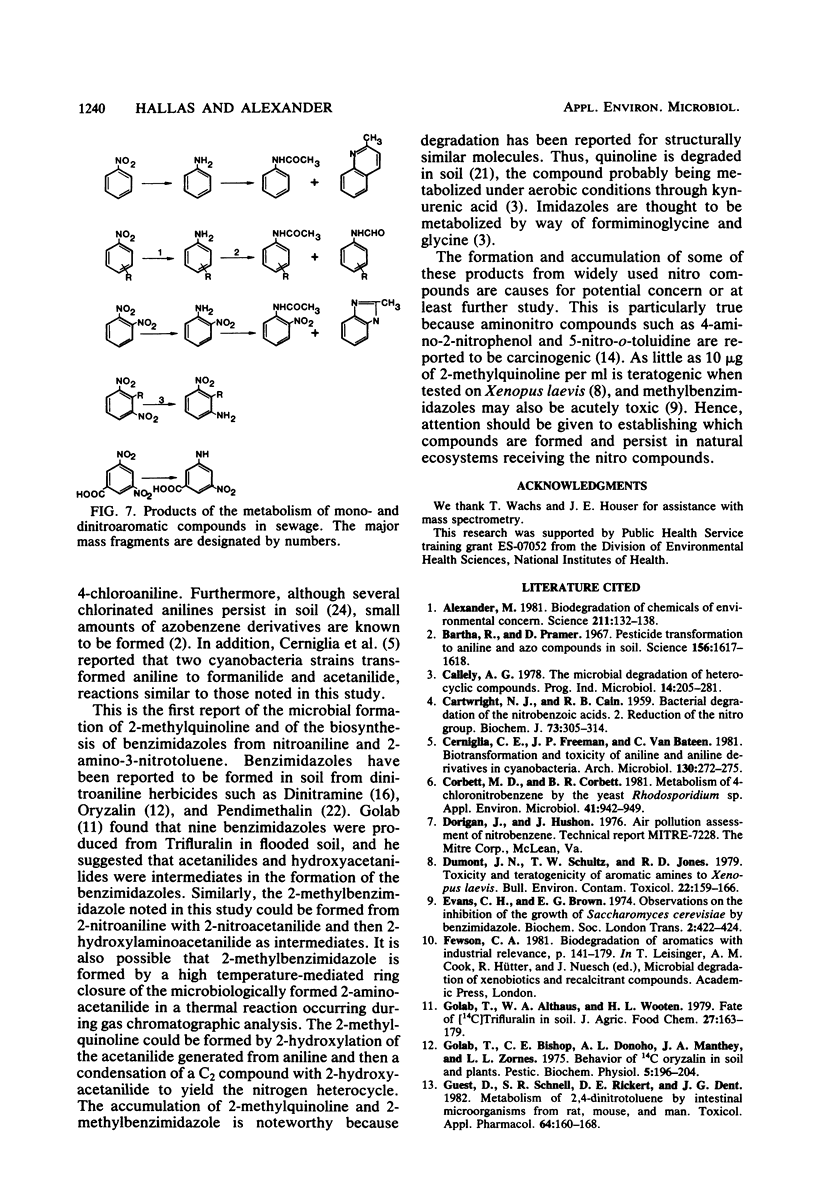
The transformation of mono- and dinitroaromatic compounds was measured in sewage effluent maintained under aerobic or anaerobic conditions. Most of the nitrobenzene, 3- and 4-nitrobenzoic acids, and 3- and 4-nitrotoluenes and much of the 1,2- and 1,3-dinitrobenzenes disappeared both in the presence and absence of oxygen. Under anaerobiosis, 2,6-dinitrotoluene and 3,5-dinitrobenzoic acid disappeared slowly, but no loss was evident in 28 days in aerated sewage. Aromatic amines did not accumulate during the aerobic decomposition of the mononitro compounds. They did appear in nonsterile, but not in sterile, sewage incubated aerobically with the dinitro compounds and anaerobically with all the chemicals. Analysis by gas chromatography and combined gas chromatography-mass spectrometry showed that aniline was formed from nitrobenzene, toluidine was formed from 3- and 4-nitrotoluenes, and aminobenzoic acid was formed from 3- and 4-nitrobenzoic acids under anaerobiosis, and that nitroaniline was formed from 1,2- and 1,3-dinitrobenzenes, aminonitrotoluene resulted from 2,6-dinitrotoluene, and aminonitrobenzoic acid was a product of 3,5-dinitrobenzoic acid under both conditions. The isomeric forms of the metabolites were not established. Aniline, 4-toluidine, and 4-aminobenzoic acid added to sewage disappeared from aerated nonsterile, but not from sterile, sewage or sewage in the absence of oxygen. 2-Nitroaniline, 2-amino-3-nitrotoluene, and 2-amino-5-nitrobenzoic acid added to sewage persisted for at least 60 days in aerobic or anaerobic conditions. Gas chromatographic and gas chromatographic-mass spectrometric analyses demonstrated that acetanilide and 2-methylquinoline were formed from aniline, 4-methylformanilide and 4-methylacetanilide were formed from 4-toluidine, 2-methylbenzimidazole was a product of 2-nitroaniline, and unidentified benzimidazoles were formed from 2-amino-3-nitrotoluene in the absence of oxygen, and that 2-nitroacetanilide and 2-methyl-6-nitroacetanilide were formed from 2-nitroaniline and 2-amino-3-nitrotoluene, respectively, in the presence or absence of oxygen. It is suggested that the transformations of widely used nitroaromatic compounds should be further studied because of the persistence and possible toxicity of products of their metabolism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M. Biodegradation of chemicals of environmental concern. Science. 1981 Jan 9;211(4478):132–138. doi: 10.1126/science.7444456. [DOI] [PubMed] [Google Scholar]
- Bartha R., Pramer D. Pesticide transformation to aniline and azo compounds in soil. Science. 1967 Jun 23;156(3782):1617–1618. doi: 10.1126/science.156.3782.1617. [DOI] [PubMed] [Google Scholar]
- CARTWRIGHT N. J., CAIN R. B. Bacterial degradation of the nitrobenzoic acids. 2. Reduction of the nitro group. Biochem J. 1959 Oct;73:305–314. doi: 10.1042/bj0730305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerniglia C. E., Freeman J. P., Van Baalen C. Biotransformation and toxicity of aniline and aniline derivatives of cyanobacteria. Arch Microbiol. 1981 Dec;130(4):272–275. doi: 10.1007/BF00425939. [DOI] [PubMed] [Google Scholar]
- Corbett M. D., Corbett B. R. Metabolism of 4-Chloronitrobenzene by the Yeast Rhodosporidium sp. Appl Environ Microbiol. 1981 Apr;41(4):942–949. doi: 10.1128/aem.41.4.942-949.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont J. N., Schultz T. W., Jones R. D. Toxicity and teratogenicity of aromatic amines to Xenopus laevis. Bull Environ Contam Toxicol. 1979 May;22(1-2):159–166. doi: 10.1007/BF02026923. [DOI] [PubMed] [Google Scholar]
- Guest D., Schnell S. R., Rickert D. E., Dent J. G. Metabolism of 2,4-dinitrotoluene by intestinal microorganisms from rat, mouse, and man. Toxicol Appl Pharmacol. 1982 Jun 15;64(1):160–168. doi: 10.1016/0041-008x(82)90335-0. [DOI] [PubMed] [Google Scholar]
- McCormick N. G., Cornell J. H., Kaplan A. M. Identification of biotransformation products from 2,4-dinitrotoluene. Appl Environ Microbiol. 1978 May;35(5):945–948. doi: 10.1128/aem.35.5.945-948.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick N. G., Feeherry F. E., Levinson H. S. Microbial transformation of 2,4,6-trinitrotoluene and other nitroaromatic compounds. Appl Environ Microbiol. 1976 Jun;31(6):949–958. doi: 10.1128/aem.31.6.949-958.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins W. J. THE CAUSE OF THE DISAPPEARANCE OF CUMARIN, VANILLIN, PYRIDINE AND QUINOLINE IN THE SOIL. Science. 1916 Dec 22;44(1147):894–895. doi: 10.1126/science.44.1147.894. [DOI] [PubMed] [Google Scholar]
- Thompson F. R., Corke C. T. Persistence and effects of some chlorinated anilines on nitrification in soil. Can J Microbiol. 1969 Jul;15(7):791–796. doi: 10.1139/m69-138. [DOI] [PubMed] [Google Scholar]
- VILLANUEVA J. R. Organic nitro compounds reduced by Nocardia V. Microbiol Esp. 1961 Jul-Sep;14:157–162. [PubMed] [Google Scholar]


