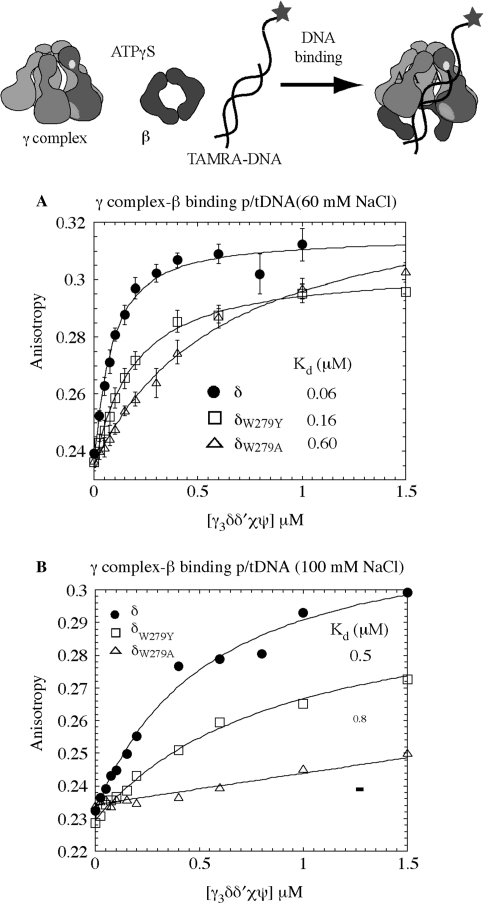
Figure 3.
The p/tDNA-binding affinity of wild-type and mutant γ complexes. (A) Fluorescence anisotropy of TAMRA-labeled 30 nt/55 nt p/t DNA (50 nM) was measured following titration with γ complex, in the presence of β and ATPγS, at 60 mM NaCl. Wild-type γ complex binds p/tDNA with high affinity (KD = 60 ± 10 nM), δW279Y-γ complex exhibits slightly lower binding and weaker affinity (KD = 159 ± 15 nM) and δW279A-γ complex has 10-fold lower affinity for p/t DNA (KD = 590 ± 80 nM). (B) At 100 mM NaCl, interaction between wild-type γ complex and p/t DNA is weaker (KD = 498 ± 12 nM) and barely detectable for δW279A-γ complex.