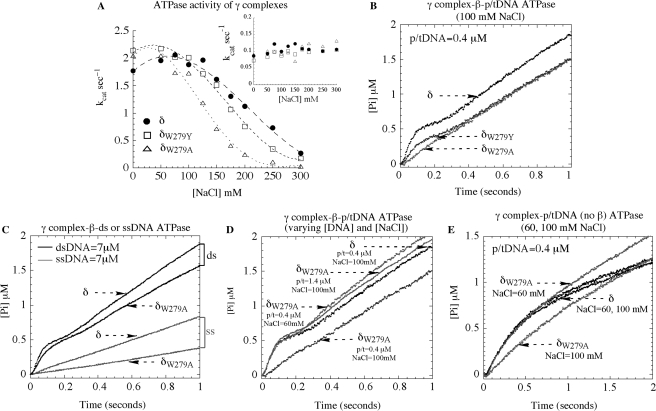
Figure 4.
ATPase activity of wild type and mutant γ complexes. (A) Steady state ATPase assays show that the β + p/tDNA-stimulated ATPase rate (kcat) of γ3δδ′χψ varies similarly with NaCl for complexes containing δ (closed circle) and δW279Y (open square), whereas the complex containing δW279A (open triangle) exhibits lower activity (the inset in A shows similar basal activity of all three complexes in the absence of DNA). (B) In pre-steady state assays at 100 mM NaCl, wild type γ complex pre-incubated with β and ATP catalyzes a rapid burst of ATP hydrolysis on mixing with 30 nt/105 nt p/tDNA. Mutant δW279Y-γ complex exhibits a decrease in the burst rate and amplitude and, notably, for δW279A-γ complex the burst phase is not detectable (final concentrations: 0.25 μM γ complex, 1 μM β, 500 μM ATP, 0.4 μM p/t DNA, and 6 μM MDCC-PBP). (C) δW279A-γ complex activity is similar to that of γ complex in the presence of duplex DNA and slightly lower in the presence of ssDNA (7 μM DNA final). (D) Reducing NaCl to 60 mM (0.4 μM p/tDNA) or raising p/tDNA concentration to 1.4 μM (100 mM NaCl) restores δW279A-γ complex burst ATPase kinetics to the same level as wild type γ complex. (E) In the absence of β, p/t DNA can stimulate a burst of ATP hydrolysis by both γ complex and δW279A-γ complex at 60 mM NaCl, but not by δW279A-γ complex at 100 mM NaCl.