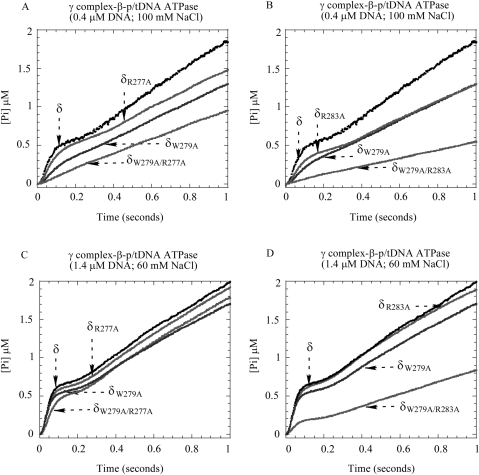
Figure 6.
The effect of δ-R277A and δ-R283A mutations on γ complex function. (A, B) At 0.4 μM p/tDNA and 100 mM NaCl, the p/tDNA-stimulated ATPase activity of δR277A-γ complex is comparable to γ complex, and δR283A-γ complex activity is slightly lower, but in the case of both double mutants, δW279A/R277A-γ complex and δW279A/R283A-γ complex, the burst phase is lost. (C, D) Under less stringent conditions (1.4 μM p/tDNA and 60 mM NaCl), the ATPase activity of the double mutants recovers somewhat (δW279A/R277A-γ complex more so than δW279A/R283A-γ complex), while the single mutants are indistinguishable from wild-type γ complex.