Figure 3. Domain structure of MMPs.
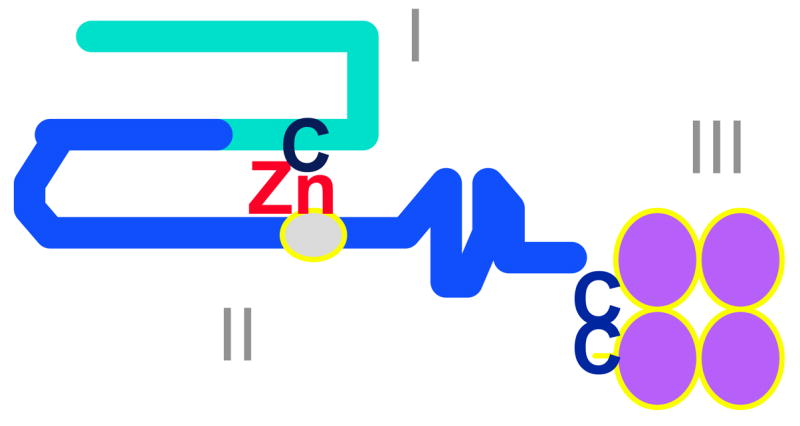
MMPs share common features including a pro-enzyme domain (I), a catalytic domain with the active site zinc bound to the HEXXHXXGXXH consensus sequence (II), and a C-terminal domain (III) which may regulate MMPs binding to their substrates and to tissue inhibitors of metalloproteinases. The catalytic zinc (Zn) atom interacts with a conserved cysteine (C) in domain I to maintain the pro-enzyme in an inactive conformation. The gelatinases have an additional domain similar to the fibronectin type II domain, which interrupts the catalytic domain. MMP-9 also has a region with homology to type V collagen (not shown).
