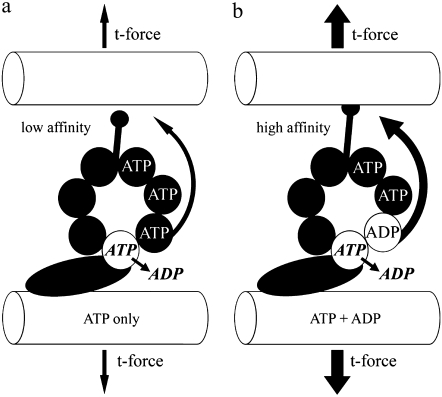
FIGURE 8.
A hypothetical mechanism relating ADP, dynein, and t-force. This illustration is based on the proposal put forth by Inoue and Shingyoji (17), and supported by our results presented in this study. The binding of ADP to a long residency regulatory site on the globular domain of the dynein head increases the force transfer to the neighboring doublet by enhancing the affinity of tubulin binding at the tip of the dynein stalk. This may be accomplished by enhanced transfer of a coordinating signal through the multiple p-loops of the AAA domain and/or an enhanced stability of the complete 3-D structure. The ultimate consequence, as relates to our work, is that the t-force that can be held and transferred through the stalk-tubule binding site is also affected, and therefore the release point to switch the dynein “off” is greatly increased. This results in the modification of the beat cycle to a higher t-force threshold for switching, consistent with what we see experimentally.