Abstract
BACKGROUND AND PURPOSE: Diffusion tensor imaging (DTI) is a sensitive technique for studying cerebral white matter. We used DTI to characterize microstructural white matter changes and their associations with cognitive dysfunction in Alzheimer disease (AD) and mild cognitive impairment (MCI).
MATERIALS AND METHODS: We studied elderly subjects with mild AD (n = 6), MCI (n = 11), or normal cognition (n = 8). A standardized clinical and neuropsychological evaluation was conducted on each subject. DTI images were acquired, and fractional anisotropy (FA), axial diffusivity (DA), and radial diffusivity (DR) of normal-appearing white matter (NAWM) in frontal, temporal, parietal, and occipital lobes were determined. These diffusion measurements were compared across the 3 groups, and significant differences were further examined for correlations with tests of cognitive function.
RESULTS: Compared with normal controls, AD subjects demonstrated decreased FA and increased DR in the temporal, parietal, and frontal NAWM and decreased DA in temporal NAWM. MCI subjects also showed decreased FA and decreased DA in temporal NAWM, with decreased FA and increased DR in parietal NAWM. Diffusion measurements showed no differences in occipital NAWM. Across all subjects, temporal lobe FA and DR correlated with episodic memory, frontal FA and DR correlated with executive function, and parietal DR significantly correlated with visuospatial ability.
CONCLUSIONS: We found evidence for functionally relevant microstructural changes in the NAWM of patients with AD and MCI. These changes were present in brain regions serving higher cortical functions, but not in regions serving primary functions, and are consistent with a hypothesized loss of axonal processes in the temporal lobe.
Structural MR imaging studies on Alzheimer disease (AD) and mild cognitive impairment (MCI) have largely focused on the brain's cortical gray matter.1–3 Although neuroimaging and pathoanatomic studies have confirmed both macroscopic and microscopic white matter changes in patients with AD and MCI, controversy exists regarding the clinical relevance of these changes and the mechanisms underlying them.4–7
By taking advantage of the anisotropic nature of water diffusion in biologic tissues, the diffusion tensor imaging (DTI) technique provides increased sensitivity for detecting ultrastructural abnormalities of white matter in vivo.8 This is true even in regions that appear normal on traditional MR imaging sequences (so called normal-appearing white matter [NAWM]).9 After generation of the diffusion tensor matrix from a series of diffusion-weighted images, 3 eigenvalues (λ1, λ2, and λ3) are calculated by matrix diagonalization. These are scalar indices, which describe water diffusion in a local (ie, voxel-specific) frame of reference and largely coincide with the geometry of local white matter tracts.10 A few investigations using DTI have reported anisotropic diffusion changes in different white matter regions in AD and MCI as measured by fractional anisotropy (FA) and mean diffusivity (MD).3,11–17 Unfortunately, FA and MD permit only a simplified description of water diffusion, and neither is particularly well suited for elucidating the pathologies underlying observed changes in water diffusion.18
Results from recent human studies and from experimental studies in animal models now permit a more analytic approach to interpreting white matter pathologies from DTI data.18–20 Accordingly, the eigenvalues (λ1, λ2, and λ3) derived by diffusion tensor matrix diagonalization are separated into components parallel (λ1) and perpendicular (λ2 and λ3) to local axon tracts. “Axial diffusivity” (DA = λ1) is defined as the magnitude of water diffusion parallel to the axon tracts within the voxel of interest. “Radial diffusivity” (DR = [λ2 + λ3]/2) describes the mean magnitude of water diffusion perpendicular to the axon tracts.18 Experimental studies show that axonal loss, as occurs in wallerian degeneration, produces a decrease in DA, with or without an increase in DR.18,19 In contrast, demyelination produces an increase in DR without a change in DA.18,20 Chronic ischemia produces increases in both DR and DA.18 These different patterns of directional diffusivity change can be used to infer underlying pathologic mechanisms affecting various brain regions in a variety of neurologic processes.
In the present study, we hypothesized that neurodegeneration produces microstructural changes in the cerebral white matter of subjects with AD and MCI. We further hypothesized that DTI could detect these changes and that the changes would correlate with measures of cognitive dysfunction. By quantifying anisotropic diffusion and examining patterns of change in directional diffusivities (DA and DR), we sought to clarify pathologic mechanisms contributing to any observed changes.
Materials and Methods
Subjects
Elderly subjects with mild AD (n = 6), MCI (n = 11), or normal cognition (n = 8) were recruited from the research registry of the Alzheimer's Disease Research Center at Case Western Reserve University. Subjects were evaluated by using a standardized clinical evaluation protocol, which included the Consortium to Establish a Registry for Alzheimer Disease (CERAD) neuropsychological battery21 and the Trail-Making Test, Parts A and B (TMT-A and TMT-B).22 All MCI subjects met the criteria for amnestic MCI of Peterson et al,23,24 5 of the single-domain type and 6 of the multiple-domain type. All AD subjects met the National Institute of Neurologic and Communicative Disorders and Stroke-Alzheimer Disease and Related Disorders Association (NINCDS-ADRDA) criteria for probable AD.25 Study procedures were approved by the local institutional review board, and informed consent was obtained from all subjects.
MR Imaging Acquisition Protocol
Conventional axial T1-weighted, T2-weighted, fluid-attenuated inversion recovery (FLAIR), and DTI sequences were acquired along the anterior/posterior commissure line with a 1.5T Magnetom Symphony MR imaging system (Siemens, Erlangen, Germany). DTI images were acquired with a single-shot pulsed-gradient, echo-planar imaging protocol (TR = 8000 ms, TE = 109 ms, FOV = 240 × 240 mm, matrix = 128 × 128, in-plane resolution = 1.875 × 1.875, section thickness = 3 mm). Diffusion gradients were applied in 12 noncollinear directions with 2 b values (0 and 1000 s/mm2). Time per DTI acquisition was 7 minutes and 6 seconds.
DTI Processing
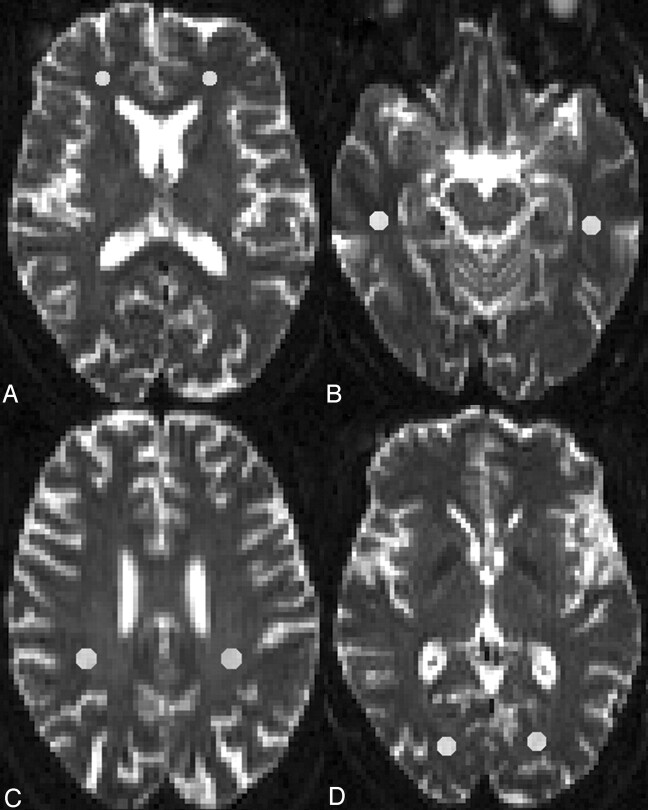
DTI dataset processing was performed by using DTIStudio (Johns Hopkins University, Baltimore, Md, http://cmrm.med.jhmi.edu), a program for the visualization and processing of diffusion MR imaging data.26 From 12 apparent diffusion coefficient maps, the maps of FA and 3 eigenvalues (λ1, λ2, λ3) were determined. NAWM was defined by normal signal intensity on standard T1-weighted, T2-weighted, and FLAIR images. Regions of interest (ROIs) were placed bilaterally on images acquired without diffusion gradients (b = 0 s/mm2) (Fig 1). Small oval ROIs of 9–16 pixels (31.64–56.25 mm2) were placed in the frontal, temporal, parietal, and occipital NAWM. Frontal lobe white matter was sampled on 5 contiguous sections, starting from the most inferior section in which the frontal horns of the lateral ventricle in both hemispheres were visible. Temporal lobe white matter was sampled on 5 contiguous sections, starting from the most inferior section in which the body of hippocampus was visible, and the ROIs were placed in white matter posterolaterally to the temporal horns of the lateral ventricle. Parietal lobe ROIs were positioned in the white matter posterior to the central sulcus on the most superior section in which the centrum semiovale was clearly visible and on the next 4 inferior sections. The occipital lobe ROIs were placed on 3 contiguous sections, starting from the most inferior section on which the occipital horn of the lateral ventricle was visible. ROIs were then superimposed on identical sections from the maps of FA and the 3 eigenvalues. FA, DA (λ1), and DR ([λ2 + λ3]/2) for each ROI were calculated. For each subject, the measures of FA, DA, and DR were averaged across all the sections of each white matter region bilaterally.
Fig 1.
Illustration of representative regions of interests positioned on T2-weighted echo-planar images (b = 0 s/mm2) in the NAWM of the frontal (A), temporal (B), parietal (C), and occipital lobes (D).
Assessment of Reliability of Measures
All the ROI placements were performed by a single rater (J.H.). Reliability of ROI placement, expressed as intraclass correlation coefficients in different white matter regions, varied from 0.88 to 0.99 for this rater.
Statistic Analyses
Data were analyzed by using the Statistical Package for Social Sciences (SPSS for Windows, Version 14.0, SPSS, Chicago, Ill). Groups were compared by using nonparametric methods, the Kruskal-Wallis test with the post hoc Mann-Whitney U test, as appropriate for the small sample sizes. Correlations were evaluated by Spearman rank correlation. Because we elected to study 4 brain regions and also designated 3 representative cognitive tests of regional brain function (specified in the “Results” section), we applied the Bonferroni correction to adjust for the effects of multiple analyses (corrected P value = .0071).
Results
There were no significant differences in age, sex, or educational level between the 3 groups. Compared with controls, AD subjects scored significantly poorer on most neuropsychological tests, whereas MCI subjects demonstrated lesser degrees of cognitive impairment (Table 1).
Table 1:
| AD (n = 6) | MCI (n = 11) | NC (n = 8) | |
|---|---|---|---|
| Age (years) | 78 (65–88) | 76 (61–87) | 73.5 (62–78) |
| Sex (female/male) | 4/2 | 5/6 | 5/3 |
| Education (years) | 13 (10–18) | 16 (11–18) | 15.5 (11–18) |
| Mini-Mental State Exam | 23 (20–26)c | 27 (24–30)c | 30 (28–30) |
| Verbal Fluency | 9.5 (8–12)cd | 17 (11–20) | 21.5 (14–32) |
| Boston Naming | 13 (7–13) | 13 (8–15) | 14.5 (13–15) |
| CP | 7.5 (6–11) | 11 (4–11) | 11 (10–11) |
| Word List Memory Total | 11 (8–21)c | 18 (9–21) | 23.5 (15–28) |
| WLDR | 2.5 (0–3)c | 4 (0–8)c | 8 (7–10) |
| Word List Recognition | 18 (16–19) | 19 (17–20) | 20 (19–20) |
| Constructional Praxis Recall | 1 (0–8) | 6 (2–11) | 11 (9–14) |
| TMT-A | 69.5 (41–254)c | 40 (22–66) | 26.5 (16–54) |
| TMT-B | 184.5 (138–300)c | 111 (53–203) | 60.5 (38–95) |
Note:—NC indicates control subjects; WLDR, Word List Delayed Recall; CP, Constructional Praxis; TMT, Trail-Making Test.
TMT-A and TMT-B are in units of seconds. All other neuropsychological units are the number of correct responses.
Median (ranges) except sex.
Statistically significant versus NC.
Statistically significant versus MCI.
FA
FA data from NAWM regions of AD, MCI, and control subjects are shown in Table 2. Significant main effects of group were revealed in white matter regional FAs in frontal (χ2 = 10.3, degrees of freedom = 2, P = .006), temporal (χ2 = 17.5, df = 2, P < .001), and parietal (χ2 = 16.5, df = 2, P < .001) lobes. Post hoc tests indicated that compared with controls, both AD and MCI subjects demonstrated decreased FA in temporal and parietal NAWM (all, P = .001), and temporal FA in AD subjects was also decreased relative to that of MCI subjects (P = .003). In frontal NAWM, AD subjects showed decreased FA compared with that of normal control subjects (P = .003). FA values in occipital NAWM showed no difference among the 3 subject groups.
Table 2:
Diffusion tensor measurements (median [ranges]) of selected NAWM regions among AD, MCI, and NC subjectsa
| AD (n = 6) | MCI (n = 11) | NC (n = 8) | |
|---|---|---|---|
| Frontal FA | 0.25 (0.22–0.29)b | 0.29 (0.25–0.38) | 0.33 (0.27–0.39) |
| Frontal DA | 1.13 (0.97–1.18) | 1.06 (0.95–1.13) | 1.07 (1.03–1.13) |
| Frontal DR | 0.78 (0.67–0.85)bc | 0.69 (0.60–0.75) | 0.64 (0.59–0.74) |
| Temporal FA | 0.38 (0.34–0.43)bc | 0.43 (0.40–0.48)b | 0.50 (0.45–0.58) |
| Temporal DA | 1.18 (1.16–1.24)b | 1.20 (1.15–1.36)b | 1.29 (1.27–1.36) |
| Temporal DR | 0.66 (0.61–0.71)b | 0.61 (0.55–0.70) | 0.57 (0.48–0.62) |
| Parietal FA | 0.38 (0.35–0.43)b | 0.40 (0.33–0.43)b | 0.46 (0.44–0.48) |
| Parietal DA | 1.14 (1.07–1.25) | 1.12 (1.05–1.18) | 1.15 (1.12–1.23) |
| Parietal DR | 0.63 (0.61–0.66)b | 0.60 (0.57–0.64)b | 0.57 (0.54–0.59) |
| Occipital FA | 0.42 (0.36–0.43) | 0.45 (0.39–0.56) | 0.45 (0.33–0.50) |
| Occipital DA | 1.19 (1.15–1.24) | 1.21 (1.06–1.42) | 1.19 (1.09–1.38) |
| Occipital DR | 0.62 (0.60–0.66) | 0.60 (0.52–0.68) | 0.59 (0.50–0.72) |
Note:—NC indicates control subjects.
DA and DR are in units of 10−3 mm2/s.
Statistically significant versus NC.
Statistically significant versus MCI.
Directional Diffusivities
Data on regional DA and DR are also shown in Table 2. In all analyses, the main effect of group was significant in frontal DR (χ2 = 10.1, df = 2, P = .007), temporal DA (χ2 = 11.3, df = 2, P = .003) and DR (χ2 = 10.4, df = 2, P = .006), and parietal DR (χ2 = 16.8, df = 2, P < .001). Post hoc analyses demonstrated that compared with normal controls, MCI subjects demonstrated decreased DA (P = .004), whereas AD subjects demonstrated decreased DA (P = .001) and increased DR (P = .001) in temporal NAWM. In parietal NAWM, both AD and MCI showed increased DR compared with controls (AD versus controls; P = .001; MCI versus controls, P < .001). In frontal NAWM, AD subjects showed more significantly increased DR than both the controls (P = .005) and the MCI (P = .007) subjects. Directional diffusivities in occipital NAWM showed no difference among the 3 subject groups.
Correlation with Cognitive Function
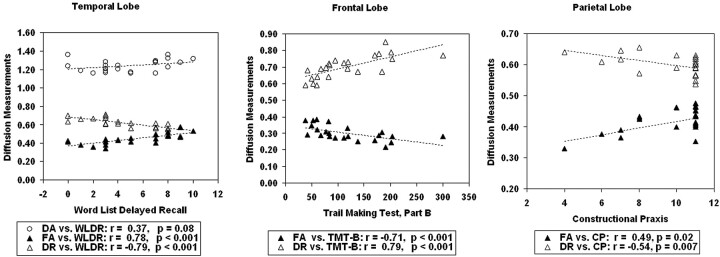
We tested for correlations between regional diffusion measurements found to be different from controls and representative cognitive tests of regional brain function. These regional diffusion measurements were FA, DR, and DA in the temporal lobe; FA and DR in the frontal lobe; and FA and DR in the parietal lobe. Our a priori cognitive test selection for temporal lobe function was Word List Delayed Recall (WLDR), for frontal lobe function was TMT-B, and for parietal lobe function was Constructional Praxis (CP) from the CERAD neuropsychological battery.21,22 Correlations were calculated by using data from all 25 subjects. Results of these analyses (Fig 2) showed that in temporal NAWM, lower FA (r = 0.78, P < .001) and higher DR (r = −0.79, P < .001) significantly correlated with poorer WLDR performance, whereas DA did not. In frontal NAWM, lower FA (r = −0.71, P < .001) and higher DR (r = 0.79, P < .001) significantly correlated with poorer TMT-B scores. In parietal NAWM, higher DR significantly correlated with poorer CP results (r = −0.54, P = .007), and FA showed a trend toward significant correlation with a P value of .02.
Fig 2.
Correlation of diffusion measurements with cognitive tests in all subjects. Poorer cognitive function is reflected by lower WLDR scores, higher TMT-B scores, and lower CP scores.
Discussion
AD is classically understood as a cortical dementia, showing prominent pathologic changes in cortical gray matter.27,28 Early microscopic studies have described the intracellular and extracellular features of AD in limbic and neocortical brain regions,29–32 and more recent MR imaging studies have demonstrated volumetric changes in corresponding gray matter regions of AD brains.1,33–36 However, relatively little attention has been directed toward abnormalities in cerebral white matter in AD.
It is reasonable to predict that abnormalities in AD would also be detectable in white matter regions. Degeneration of perikarya in the cerebral cortex could secondarily produce axonal loss in the subjacent white matter, because intercortical and extracortical projection fibers are lost through the degenerative process.37 Cerebrovascular disease is often present in elderly patients with AD, and ischemic mechanisms could also contribute to produce abnormalities in cerebral white matter.38 Other processes that could be hypothesized to produce white matter changes in AD include primary and secondary demyelination and reactive gliosis.39,40
FA is an imaging marker commonly used to study microstructural white matter abnormalities in various pathologic states.3,8–17 Results of decreased FA in the present study suggest microstructural degradation of cerebral white matter in AD and MCI. The observed differences in FA follow a clear regional gradient, with the greatest changes seen in the temporal lobe, followed by the parietal lobe, then the frontal lobe, and no changes compared with controls in the occipital lobe. These results are consistent with most prior studies measuring FA in AD and MCI. Collectively, these studies suggest that the distribution of white matter abnormalities in AD and MCI is inhomogeneous, showing concentrations in regions connected with association cortices (posterior cingulum fibers, corpus callosum, and temporal, frontal, and parietal lobe white matter) and largely sparing regions serving motor (internal capsule) or visual (optic radiation) functions.3,11–17
Because FA is a relatively simple descriptor of water diffusivity, it provides limited information useful for determining specific brain pathologies underlying changes in water diffusion. We, therefore, further studied changes in directional diffusivities (DA and DR) in the NAWM of AD and MCI subjects. This approach revealed reductions in DA and increases in DR in the white matter of subjects with MCI and AD. This pattern of change in directional diffusivities (↓DA, ↑DR) is highly suggestive of axonal loss as occurs with wallerian degeneration.18,19 We observed this axonal loss pattern in the temporal white matter of subjects with MCI and AD. This finding is consistent with the known propensity for early degeneration of temporal lobe structures such as the hippocampal formation, parahippocampal gyrus, and entorhinal cortex in AD.1,3,30–31,33–34 Furthermore, because DA is more strongly related to axonal pathology and DR is more strongly related to demyelination,18,19 the changes in temporal white matter directional diffusivities of MCI (↓DA) may reflect early axonal damage, and the directional diffusivity changes in temporal white matter of AD (↓DA, ↑DR) may reflect complete loss of myelinated axons as would be seen in wallerian degeneration. Lesser changes in directional diffusivities were also observed in the frontal and parietal white matter of MCI and AD subjects but were not observed in the occipital white matter. This apparent regional gradient in white matter changes closely parallels the known distribution of regional neuropathology in AD.27 Our results are also consistent with an earlier DTI study in a transgenic mouse model of β-amyloid deposition.41 Using the APPsw transgenic mouse (Tg2576), Sun et al41 demonstrated significant decreased DA with largely unaltered DR in the white matter of a group of transgenic mice, compared with a group of age-matched wild-type mice.
The changes in FA and directional diffusivities observed in our subjects with MCI and AD appear to have functional relevance. First, these changes were identified in brain regions serving higher cortical functions (temporal, parietal, and frontal lobes) but not in regions serving primary functions (occipital lobe). Furthermore, when we examined the relationships of significant changes in diffusion measurements to subjects’ performance on cognitive tests, important correlations surfaced. Key findings are the correlation between diffusion measurements in the temporal lobes and episodic memory function, the correlation between frontal lobe diffusion measurements and executive function, and the correlation between parietal lobe diffusion measurements and visuospatial ability. These results agree with our current understanding of relationships between brain regions and domains of cognitive function and reveal a pattern of poorer cognitive test performance in association with loss of anisotropic diffusion.42–45
Our study has several limitations. The small sample size limits the power of this pilot investigation. This limitation probably contributed to the observed variability in results across different white matter regions. Although our findings within the temporal white matter support a hypothesis of axonal degeneration, we did not observe uniformly consistent findings in the parietal or frontal white matter. Another possible explanation for different findings in various brain regions could be the opposing effects of secondary pathologies. For example, chronic cerebral ischemia is known to produce increases in both DA and DR.18 Although increases in DR from degenerative and vascular mechanisms would combine in an additive fashion, decreased DA from axonal degeneration would be opposed by increased DA from concomitant vascular pathology. Because vascular pathology commonly affects the frontal and parietal white matter to a greater extent than it affects temporal or occipital white matter,46,47 concomitant cerebrovascular disease could preferentially obscure the effects of neurodegeneration on DA in the frontal and parietal lobes. Finally, geometric imaging distortions due to eddy currents may impact the accuracy of our ROI positioning. Newer MR imaging techniques, using double-refocusing radio-frequency pulses with bipolar gradients to reduce eddy current effects, might reduce this concern in future studies.17 Furthermore, although we applied 12 diffusion gradient directions to acquire DTI images (rather than the minimum-required 6 directions used in many previous studies), recent advances in DTI techniques suggest that increasing numbers of diffusion-encoding gradient directions (optimally 20 or more directions) and using isotropic voxels may improve estimation of the diffusion tensor.48
One prior study measured directional diffusivities in the white matter of AD subjects.49 In that study, DR in the periventricular frontal white matter was increased compared that of with normal control subjects, without a corresponding decrease in DA. The authors proposed that the observed changes in directional diffusivities may reflect myelin-related pathologies consistent with a retrogenesis theory of AD.50 However, because that study did not exclude white matter hyperintensities on T2-weighted or FLAIR images when defining ROIs, their findings could be explained by the opposing effects of neurodegeneration and concomitant vascular pathology on DA in the frontal periventricular region. Further studies with large numbers of MCI and AD subjects, wherein vascular aspects of regional cerebral pathology could be included in the analyses, may help to resolve such complexities.
Conclusions
In this study, we found evidence for functionally relevant microstructural changes in the NAWM of patients with AD and MCI. These changes were present in brain regions serving higher cortical functions (frontal, temporal, and parietal lobes), but not in regions serving primary functions (occipital lobe). These preliminary observations support the view that DTI is a useful in vivo tool for identifying microstructural cerebral pathology in MCI and AD and provide preliminary evidence suggesting axonal degeneration as an underlying mechanism for such changes in the NAWM of the temporal lobe.
Acknowledgments
We thank Sherye Sirrel and Ellen Grady for excellence in recruiting participants for this study.
Footnotes
This work was supported by the National Institute on Aging (grant P50AG08012), the Margaret Askew Fund for Alzheimer's Research, and the R.L. Cates Mount Research Fund.
References
- 1.Jack CR Jr, Petersen RC, Xu YC, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology 1999;52:1397–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chetelat G, Baron J. Early diagnosis of Alzheimer's disease: contribution of structural neuroimaging. Neuroimage 2003;18:525–41 [DOI] [PubMed] [Google Scholar]
- 3.Ramani A, Jensen JH, Helpern JA. Quantitative MR imaging in Alzheimer disease. Radiology 2006;241:26–44 [DOI] [PubMed] [Google Scholar]
- 4.Englund E. Neuropathology of white matter changes in Alzheimer's disease and vascular dementia. Dement Geriatr Cogn Disord 1998;9 (suppl 1):6–12 [DOI] [PubMed] [Google Scholar]
- 5.Scheltens P, Barkhof F, Leys D, et al. Histopathologic correlates of white matter changes on MRI in Alzheimer's disease and normal aging. Neurology 1995;45:883–88 [DOI] [PubMed] [Google Scholar]
- 6.Bracco L, Piccini C, Moretti M, et al. Alzheimer's disease: role of size and location of white matter changes in determining cognitive deficits. Dement Geriatr Cogn Disord 2005;20:358–66 [DOI] [PubMed] [Google Scholar]
- 7.Kono I, Mori S, Nakajima K, et al. Do white matter changes have clinical significance in Alzheimer's disease? Gerontology 2004;50:242–46 [DOI] [PubMed] [Google Scholar]
- 8.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 1996;111:209–19 [DOI] [PubMed] [Google Scholar]
- 9.Werring DJ, Clark CA, Barker GJ, et al. Diffusion tensor imaging of lesions and normal-appearing white matter in multiple sclerosis. Neurology 1999;52:1626–32 [DOI] [PubMed] [Google Scholar]
- 10.Pierpaoli C, Jezzard P, Basser PJ, et al. Diffusion tensor MR imaging of the human brain. Radiology 1996;201:637–48 [DOI] [PubMed] [Google Scholar]
- 11.Bozzali M, Falini A, Franceschi M, et al. White matter damage in Alzheimer's disease assessed in vivo using diffusion tensor magnetic resonance imaging. J Neurol Neurosurg Psychiatry 2002;72:742–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fellgiebel A, Wille P, Muller MJ, et al. Ultrastructural hippocampal and white matter alterations in mild cognitive impairment: a diffusion tensor imaging study. Dement Geriatr Cogn Disord 2004;18:101–08 [DOI] [PubMed] [Google Scholar]
- 13.Naggara O, Oppenheim C, Rieu D, et al. Diffusion tensor imaging in early Alzheimer's disease. Psychiatry Res 2006;146:243–49 [DOI] [PubMed] [Google Scholar]
- 14.Takahashi S, Yonezawa H, Takahashi J, et al. Selective reduction of diffusion anisotropy in white matter of Alzheimer disease brains measured by 3.0 Tesla magnetic resonance imaging. Neurosci Lett 2002;332:45–8 [DOI] [PubMed] [Google Scholar]
- 15.Taoka T, Iwasaki S, Sakamoto M, et al. Diffusion anisotropy and diffusivity of white matter tracts within the temporal stem in Alzheimer disease: evaluation of the “tract of interest” by diffusion tensor tractography. AJNR Am J Neuroradiol 2006;27:1040–45 [PMC free article] [PubMed] [Google Scholar]
- 16.Medina D, DeToledo-Morrell L, Urresta F, et al. White matter changes in mild cognitive impairment and AD: a diffusion tensor imaging study. Neurobiol Aging 2006;27:663–72 [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Schuff N, Jahng GH, et al. Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology 2007;68:13–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song SK, Sun SW, Ju WK, et al. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 2003;20:1714–22 [DOI] [PubMed] [Google Scholar]
- 19.Pierpaoli C, Barnett A, Pajevic S, et al. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage 2001;13:1174–85 [DOI] [PubMed] [Google Scholar]
- 20.Song SK, Sun SW, Ramsbottom MJ, et al. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 2002;17:1429–36 [DOI] [PubMed] [Google Scholar]
- 21.Welsh KA, Butters N, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology 1994;44:609–14 [DOI] [PubMed] [Google Scholar]
- 22.Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. 2nd ed. Tucson: Neuropsychology Press;1993
- 23.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol 2001;58:1985–92 [DOI] [PubMed] [Google Scholar]
- 24.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256:183–94 [DOI] [PubMed] [Google Scholar]
- 25.Mckhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Forces on Alzheimer's Disease. Neurology 1984;34:939–44 [DOI] [PubMed] [Google Scholar]
- 26.Jiang H, van Zijl PC, Kim J, et al. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed 2006;81:106–16 [DOI] [PubMed] [Google Scholar]
- 27.Braak E, Griffing K, Arai K, et al. Neuropathology of Alzheimer's disease: what is new since A. Alzheimer? Eur Arch Psychiatry Clin Neurosci 1999;249 (suppl 3):14–22 [DOI] [PubMed] [Google Scholar]
- 28.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 1991;41:479–86 [DOI] [PubMed] [Google Scholar]
- 29.Hauw JJ, Duyckaerts C, Delaere P, et al. Alzheimer's disease: neuropathological and etiological data. Biomed Pharmacother 1989;43:469–82 [DOI] [PubMed] [Google Scholar]
- 30.Pearson RC, Esiri MM, Hiorns RW, et al. Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer's disease. Proc Natl Acad Sci U S A 1985;82:4531–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis DA, Campbell MJ, Terry RD, et al. Laminar and regional distributions of neurofibrillary tangles and neuritic plaques in Alzheimer's disease: a quantitative study on visual and auditory cortices. J Neurosci 1987;7:1799–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Price JL, Davis PB, Morris JC, et al. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer's disease. Neurobiol Aging 1991;12:295–312 [DOI] [PubMed] [Google Scholar]
- 33.Juottonen K, Laakso MP, Insausti R, et al. Volumes of the entorhinal and perirhinal cortices in Alzheimer's disease. Neurobiol Aging 1998;19:15–22 [DOI] [PubMed] [Google Scholar]
- 34.Krasuski JS, Alexander GE, Horwitz B, et al. Volumes of medial temporal lobe structures in patients with Alzheimer's disease and mild cognitive impairment (and in healthy controls). Biol Psychiatry 1998;43:60–68 [DOI] [PubMed] [Google Scholar]
- 35.Fox NC, Scahill RI, Crum WR, et al. Correlation between rates of brain atrophy and cognitive decline in AD. Neurology 1999;52:1687–89 [DOI] [PubMed] [Google Scholar]
- 36.Karas GB, Burton EJ, Rombouts SA, et al. A comprehensive study of gray matter loss in patients with Alzheimer's disease using optimized voxel-based morphometry. Neuroimage 2003;18:895–907 [DOI] [PubMed] [Google Scholar]
- 37.Leys D, Pruvo JP, Parent M, et al. Could wallerian degeneration contribute to “leuko-araiosis” in subjects free of any vascular disorder? J Neurol Neurosurg Psychiatry 1991;54:46–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brilliant M, Hughes L, Anderson D, et al. Rarefied white matter in patients with Alzheimer disease. Alzheimer Dis Assoc Disord 1995;9:39–46 [DOI] [PubMed] [Google Scholar]
- 39.Bartzokis G, Sultzer D, Lu PH, et al. Heterogeneous age-related breakdown of white matter structural integrity: implications for cortical “disconnection” in aging and Alzheimer's disease. Neurobiol Aging 2004;25:843–51 [DOI] [PubMed] [Google Scholar]
- 40.Brun A, Englund E. A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Ann Neurol 1986;19:253–62 [DOI] [PubMed] [Google Scholar]
- 41.Sun SW, Song SK, Harms MP, et al. Detection of age-dependent brain injury in a mouse model of brain amyloidosis associated with Alzheimer's disease using magnetic resonance diffusion tensor imaging. Exp Neurol 2005;191:77–85 [DOI] [PubMed] [Google Scholar]
- 42.Duan JH, Wang HQ, Xu J, et al. White matter damage of patients with Alzheimer's disease correlated with the decreased cognitive function. Surg Radiol Anat 2006;28:150–56 [DOI] [PubMed] [Google Scholar]
- 43.Fellgiebel A, Muller MJ, Wille P, et al. Color-coded diffusion-tensor-imaging of posterior cingulate fiber tracts in mild cognitive impairment. Neurobiol Aging 2005;26:1193–98. Epub 2005 Jan 12 [DOI] [PubMed] [Google Scholar]
- 44.Yoshiura T, Mihara F, Ogomori K, et al. Diffusion tensor in posterior cingulate gyrus: correlation with cognitive decline in Alzheimer's disease. Neuroreport 2002;13:2299–302 [DOI] [PubMed] [Google Scholar]
- 45.Rose SE, Chen F, Chalk JB, et al. Loss of connectivity in Alzheimer's disease: an evaluation of white matter tract integrity with colour coded MR diffusion tensor imaging. J Neurol Neurosurg Psychiatry 2000;69:528–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Leeuw FE, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population-based magnetic resonance imaging study—The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry 2001;70:9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshita M, Fletcher E, Harvey D, et al. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology 2006;67:2192–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ni H, Kavcic V, Zhu T, et al. Effects of number of diffusion gradient directions on derived diffusion tensor imaging indices in human brain. AJNR Am J Neuroradiol 2006;27:1776–81 [PMC free article] [PubMed] [Google Scholar]
- 49.Choi SJ, lim KO, Monteiro I, et al. Diffusion tensor imaging of frontal white matter microstructure in early Alzheimer's disease: a preliminary study. J Geriatr Psychiatry Neurol 2005;18:12–19 [DOI] [PubMed] [Google Scholar]
- 50.Reisberg B, Franssen EH, Hasan SM, et al. Retrogenesis: clinical, physiologic, and pathologic mechanisms in brain aging, Alzheimer's and other dementing processes. Eur Arch Psychiatry Clin Neurosci 1999;249 (suppl 3):28–36 [DOI] [PubMed] [Google Scholar]