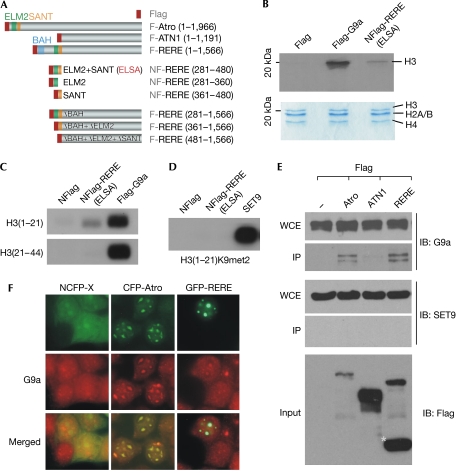
Figure 1.
Arginine–glutamic acid dipeptide repeats protein exerts histone methylation activity and associates with G9a. (A) Diagram showing the Flag-tagged Atrophin (Atro) proteins used in immunoprecipitation experiments. The conserved BAH (bromo-adjacent homology), ELM2 (EGL-27 and MTA1 homology 2) and SANT (SWI3/ADA2/N-CoR/TFIII-B) domains in each protein are highlighted in different colours. (B) Histone methylation (HMT) assays were performed on recombinant histone octamers to determine whether the ELM2–SANT domains of arginine–glutamic acid dipeptide repeats protein (RERE) mediate HMT activity. The identity of each histone is determined by its size in the Coomassie blue-stained gel; G9a, a known histone H3-specific histone methyltransferase (HMTase), was used as a control. (C,D) HMT assays were performed on the indicated peptides to determine which specific lysine residue is a target of the REREELSA complex. Recombinant SET9, a known H3K4 HMTase, was used as a control. (E) Western blot experiments were performed on the indicated immunoprecipitation products to test whether G9a is associated with Atro proteins. SET9 was used as a negative control. A cleaved product of RERE is marked with an asterisk. (F) Immunostaining experiments were performed on cells expressing green fluorescent protein (GFP)-tagged RERE or cyan fluorescent protein (CFP)-tagged Atro to determine whether endogenous G9a is recruited to the RERE/Atro-mediated nuclear foci. Nuclear localization signal-tagged CFP (NCFP) was used as a control. ELSA, ELM2+SANT domains; F, Flag; H3K9met2, dimethylated H3K9; IB, immunoblot; IP, immunoprecipitation; NF, nuclear localization signal-tagged Flag; WCE, whole-cell extract.