Abstract
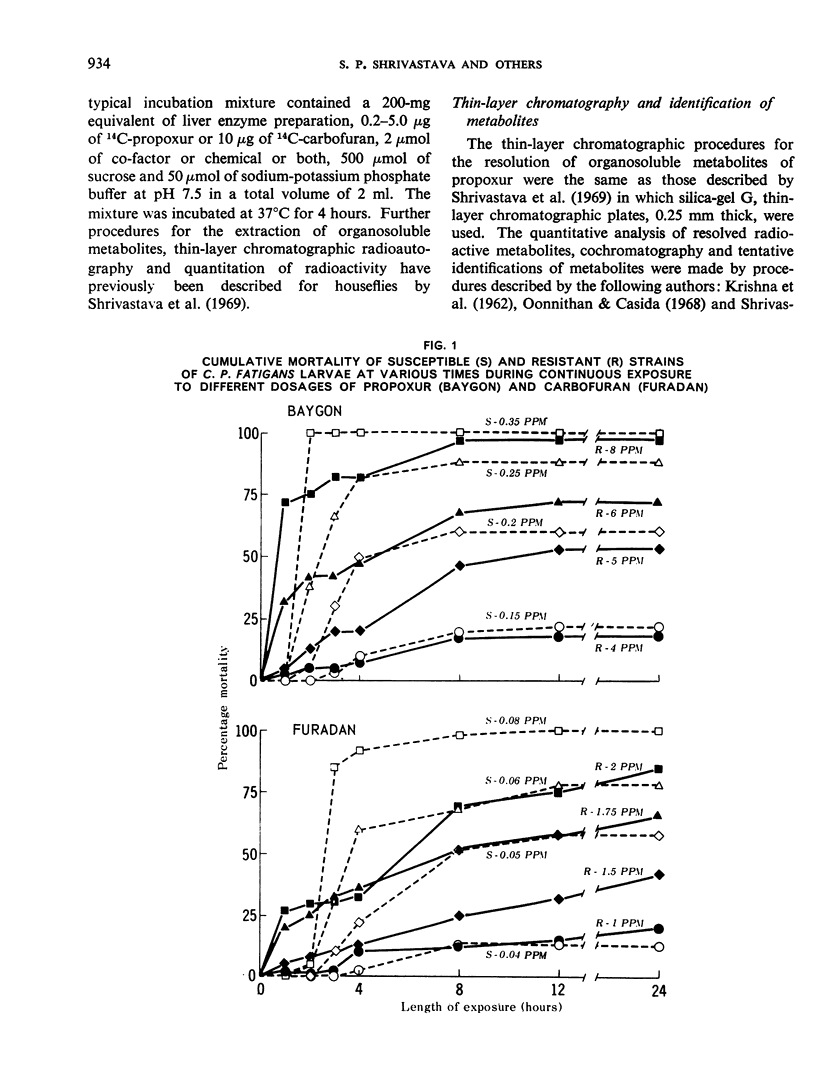
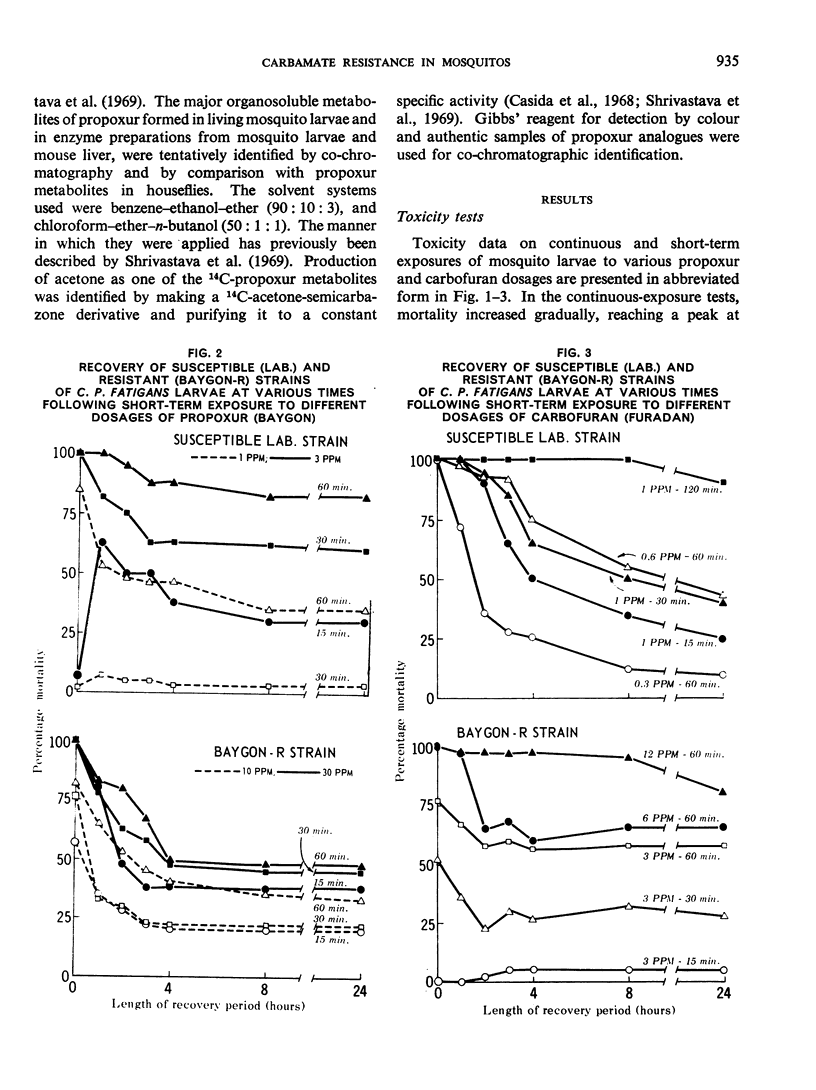
Toxicity tests on Culex pipiens fatigans with propoxur (o-isopropoxyphenyl methylcarbamate) and carbofuran (2,2-dimethyl-2,3-dihydrobenzofuranyl-7-methylcarbamate) indicated that both compounds are fast-acting insecticides. Transfer of treated larvae to fresh water results in their partial recovery from knockdown.
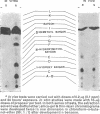
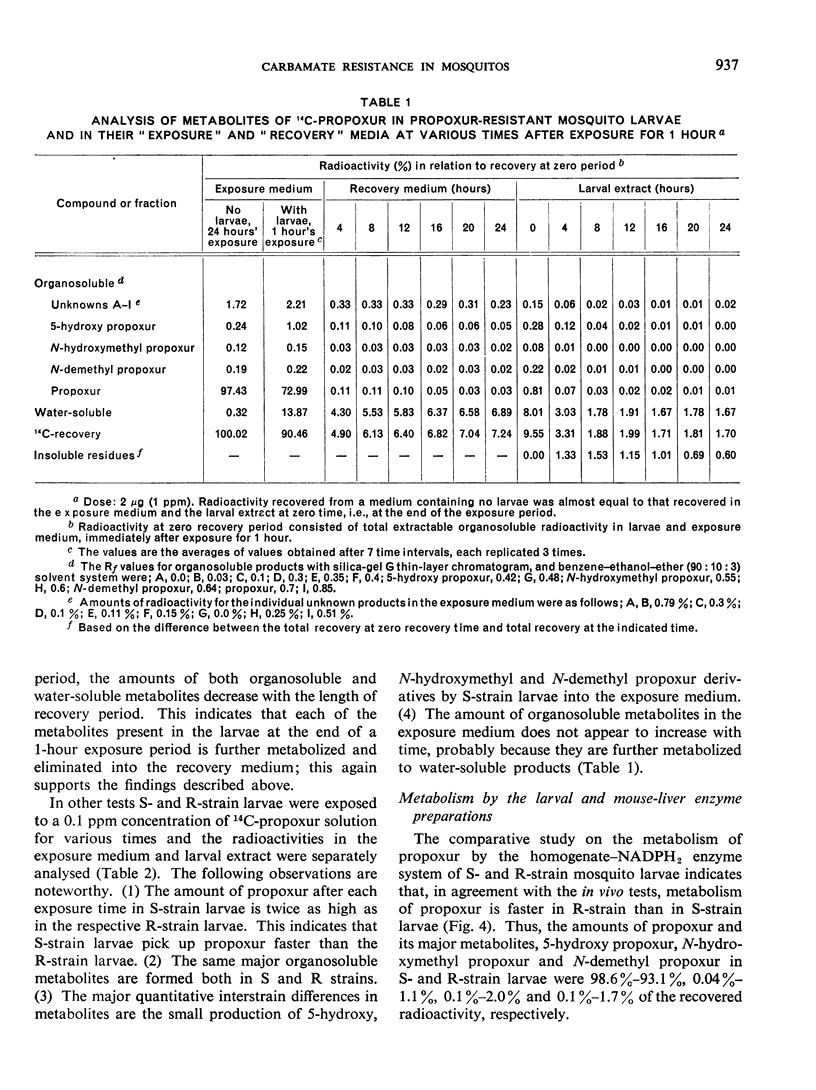
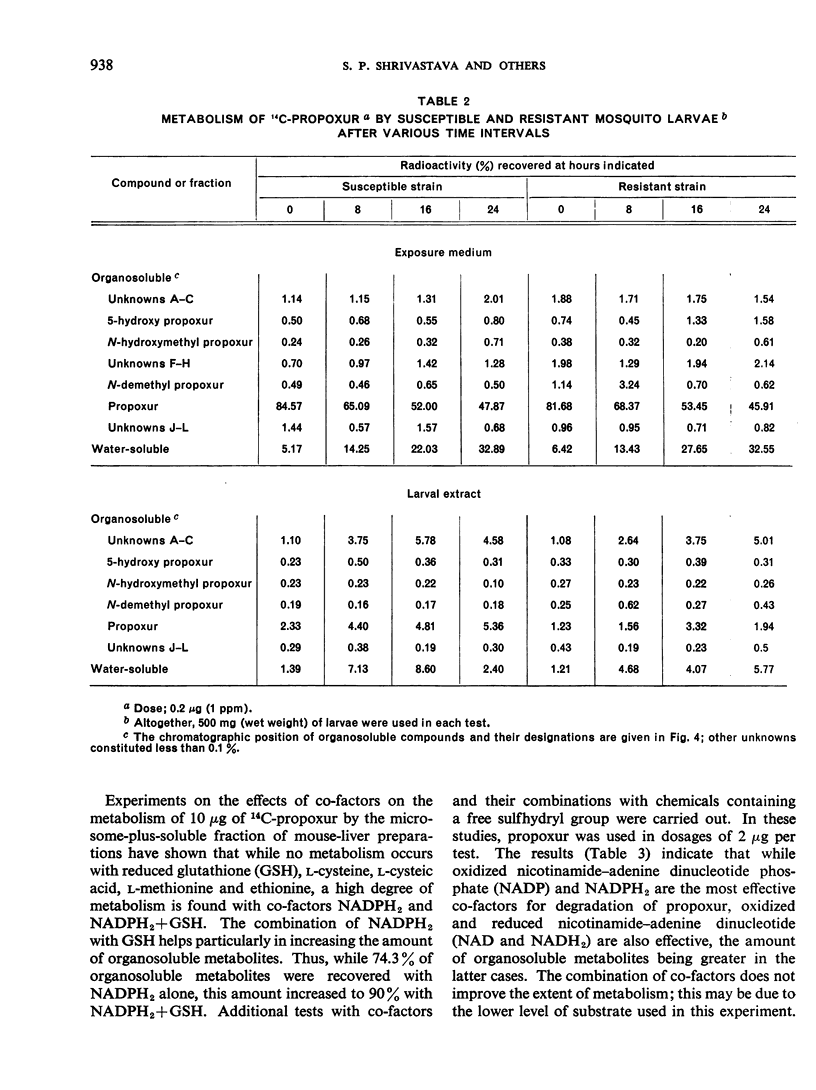
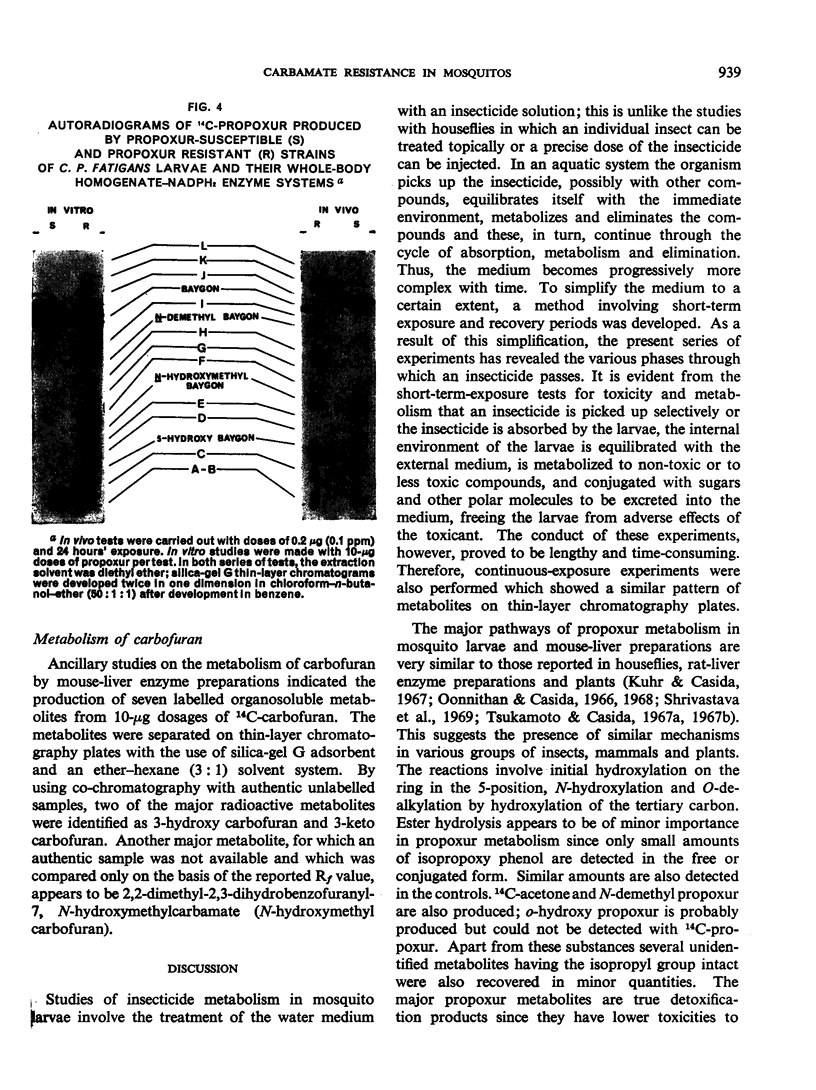
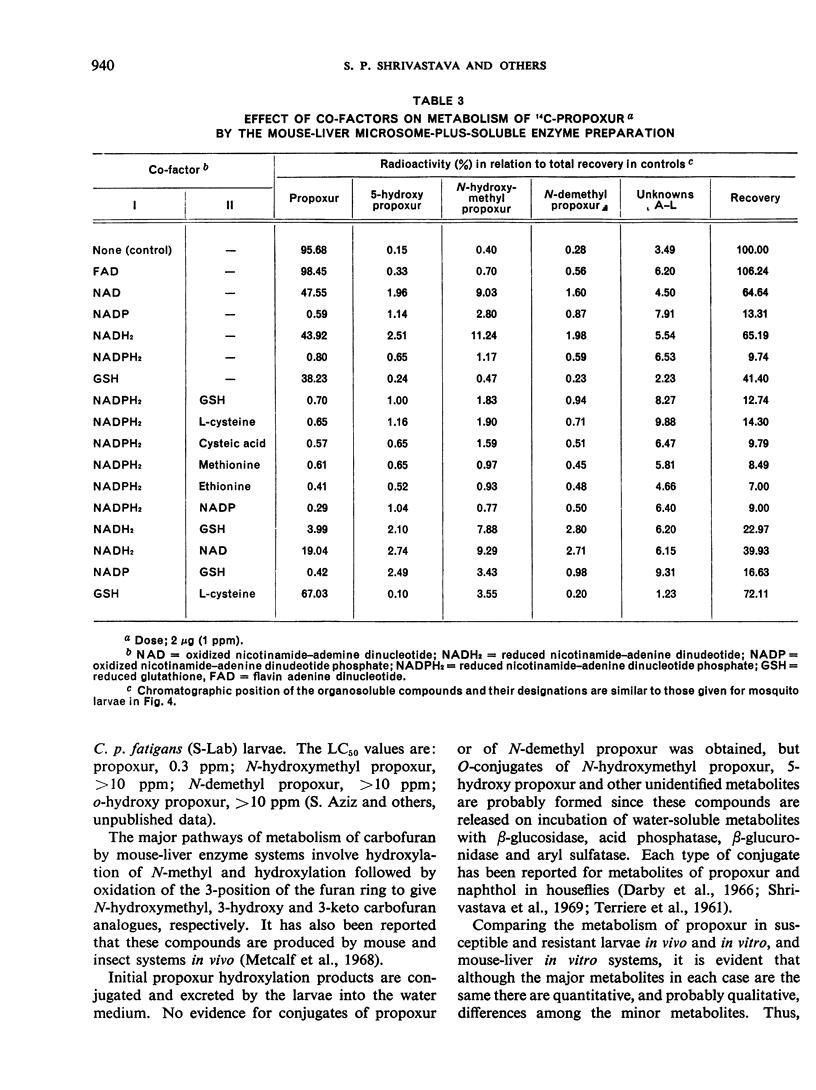
Propoxur is metabolized by resistant and susceptible larvae by their homogenate-reduced nicotinamide—adenine dinucleotide phosphate (NADPH2) enzyme system and by the microsome-plus-soluble fraction of mouse-liver extracts to at least 10 organosoluble metabolites with the isopropoxy group intact. The major metabolites, which are primarily hydroxylation products or the result of degradation of these products, have tentatively been identified as: acetone plus o-hydroxyphenyl methylcarbamate, 2-isopropoxy-5-hydroxyphenyl methylcarbamate, 2-isopropoxyphenyl carbamate, and 2-isopropoxyphenyl N-hydroxymethylcarbamate. Upon incubation of water-soluble products from treated larvae with β-glucosidase, β-glucuronidase, aryl sulfatase and acid phosphatase, the conjugates are hydrolysed, liberating mainly hydroxylated carbamates.
The results indicate that slower absorption as well as faster detoxification by hydroxylation mechanisms, together with conjugation with polar molecules and elimination, are major factors in resistance of mosquito larvae to substituted-aryl methylcarbamate insecticides.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casida J. E., Shrivastava S. P., Esaac E. G. Selective recovery of volatile products from house flies treated with radioactive insecticide chemicals and synergists. J Econ Entomol. 1968 Oct;61(5):1339–1344. doi: 10.1093/jee/61.5.1339. [DOI] [PubMed] [Google Scholar]
- Georghiou G. P., Metcalf R. L., Gidden F. E. Carbamate-resistance in mosquitos. Selection of Culex pipiens fatigans Wiedemann (=C. quinquefasciatus Say) for resistance to Baygon. Bull World Health Organ. 1966;35(5):691–708. [PMC free article] [PubMed] [Google Scholar]
- TERRIERE L. C., BOOSE R. B., ROUBAL W. T. The metabolism of naphthalene and 1-naphthol by houseflies and rats. Biochem J. 1961 Jun;79:620–623. doi: 10.1042/bj0790620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto M., Casida J. E. Albumin enhancement of oxidative metabolism of methylcarbamate insecticide chemicals by the house fly microsome-NADPH2 system. J Econ Entomol. 1967 Apr;60(2):617–619. doi: 10.1093/jee/60.2.617. [DOI] [PubMed] [Google Scholar]
- Tsukamoto M., Shrivastava S. P., Casida J. E. Biochemical genetics of house fly resistance to carbamate insecticide chemicals. J Econ Entomol. 1968 Feb;61(1):50–55. doi: 10.1093/jee/61.1.50. [DOI] [PubMed] [Google Scholar]