Abstract
The lack of reliable laboratory methods of determining the antigenicity of inactivated influenza virus vaccines prompted a reinvestigation of the reproducibility of the tests used for measuring the antigenic content of influenza vaccines, namely, the CCA and mouse potency tests.
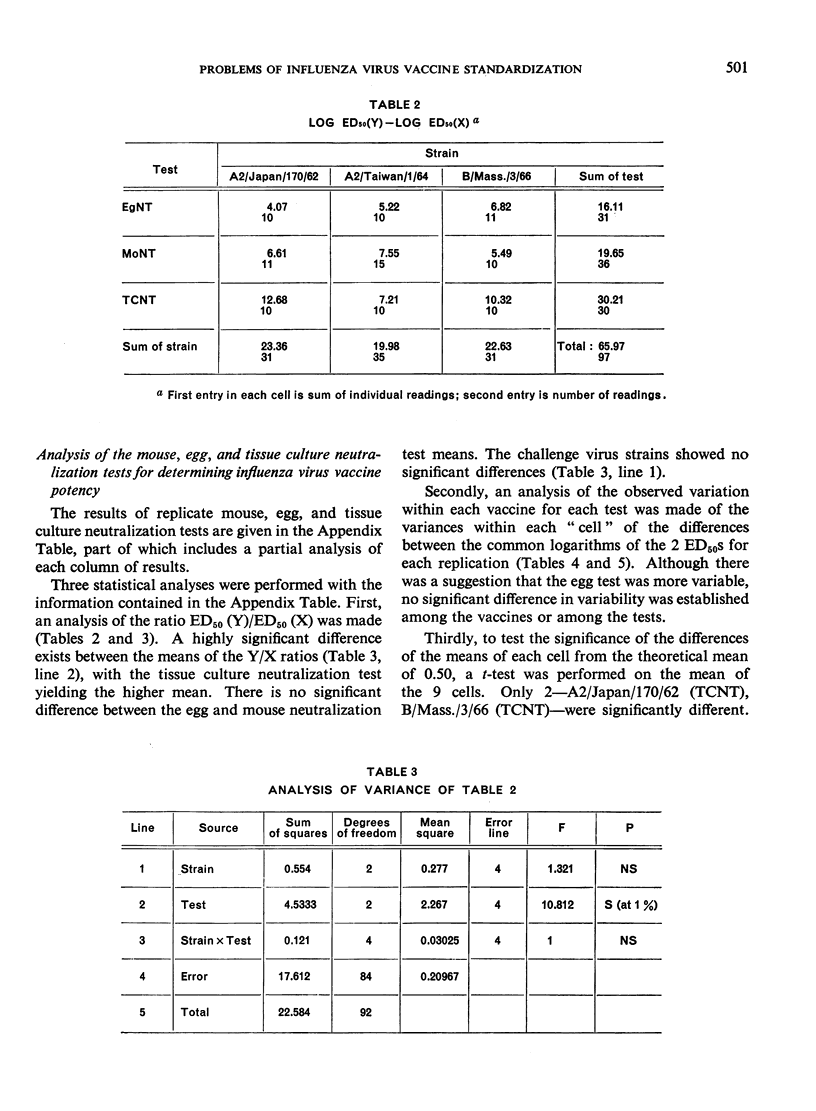
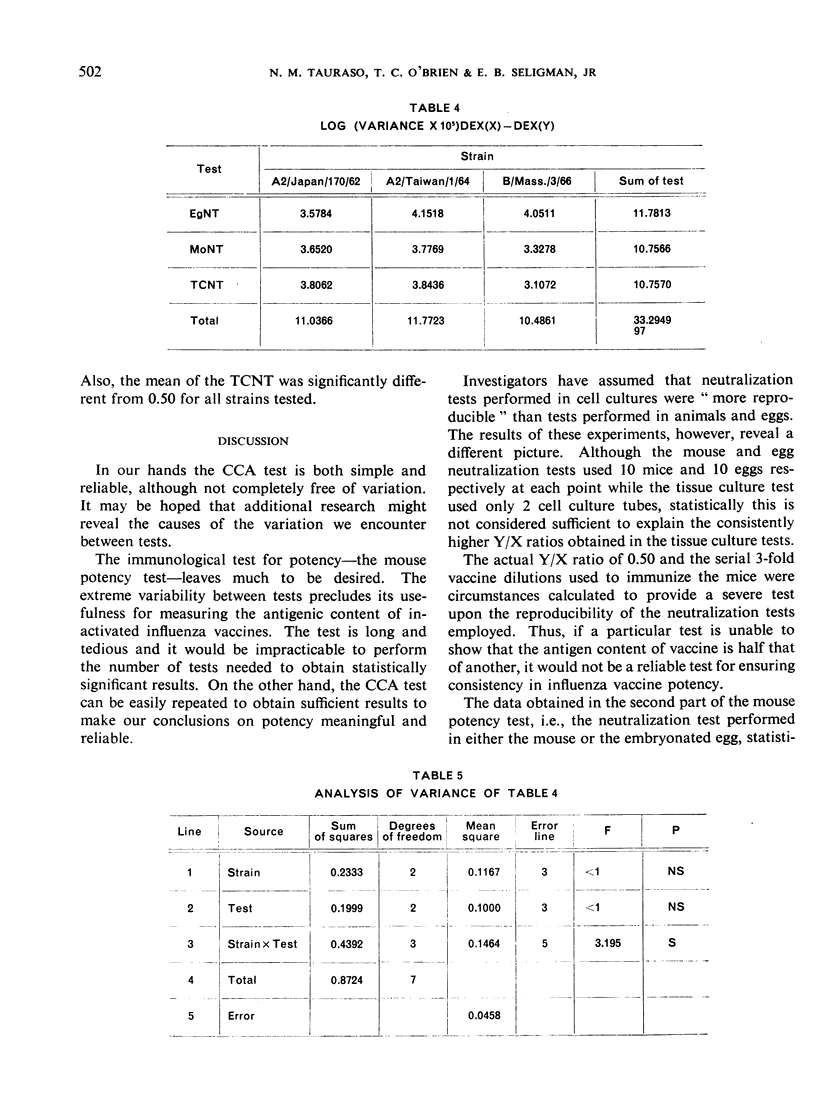
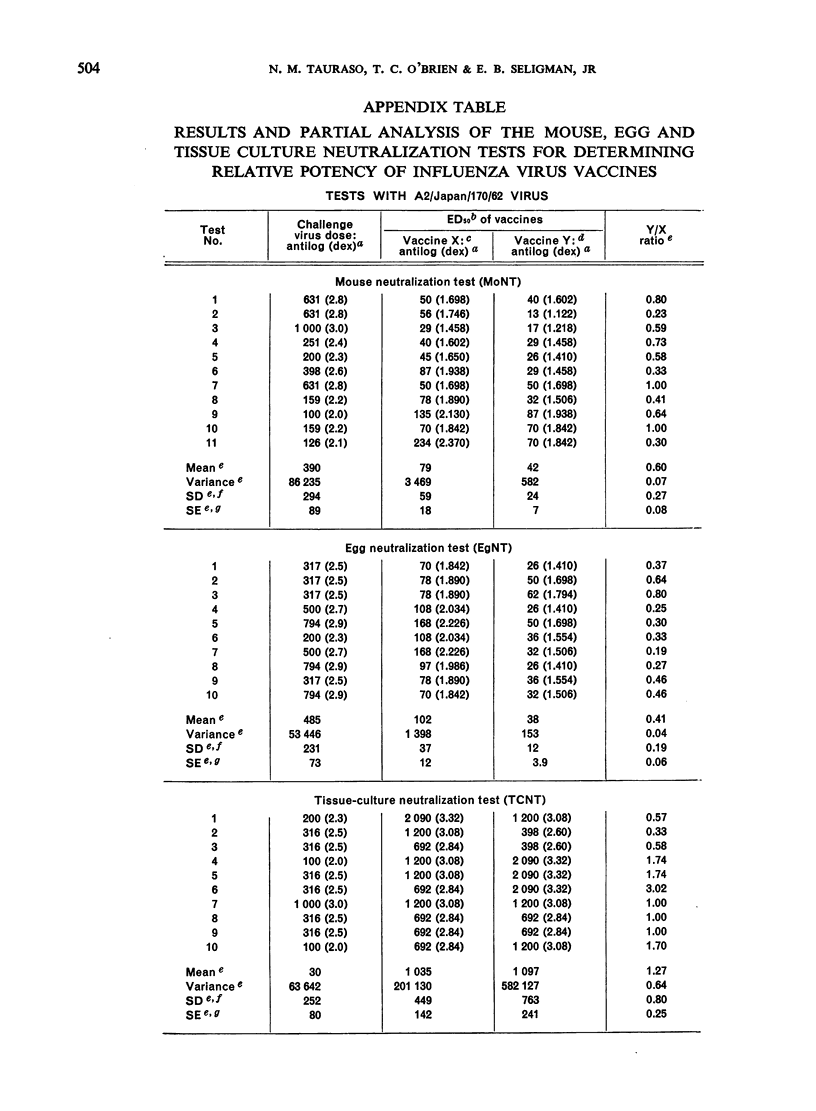
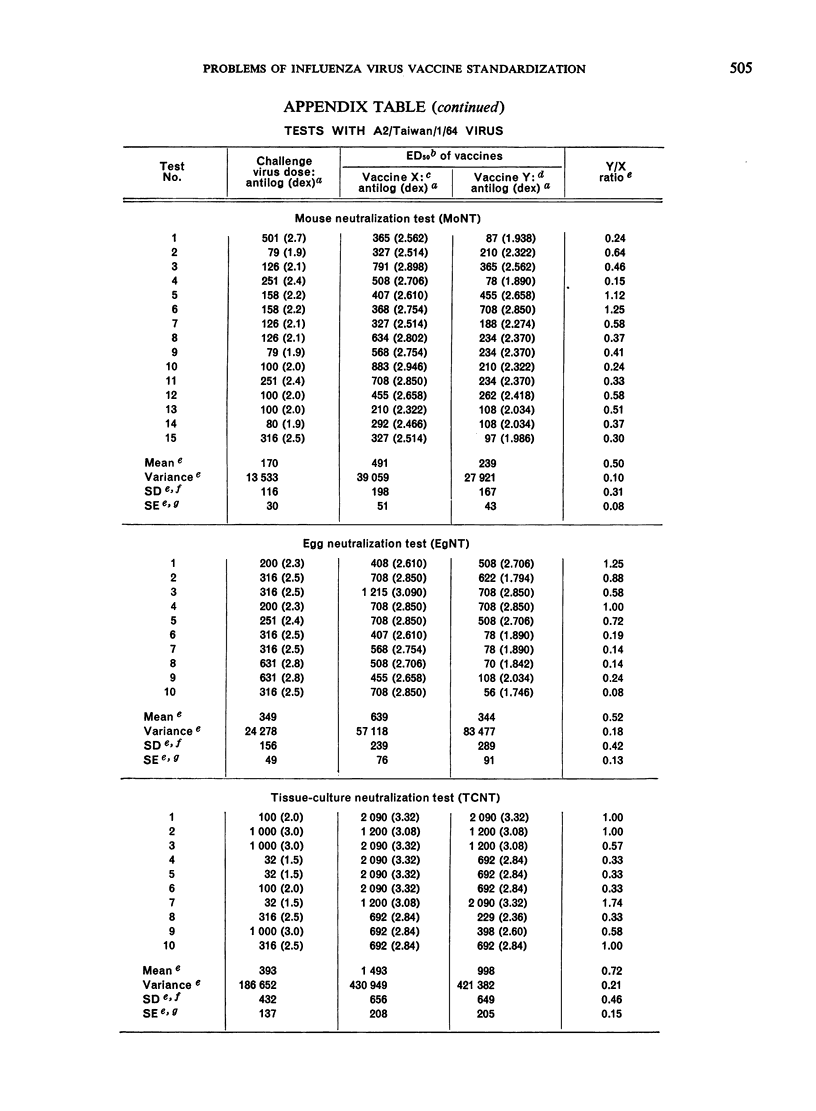
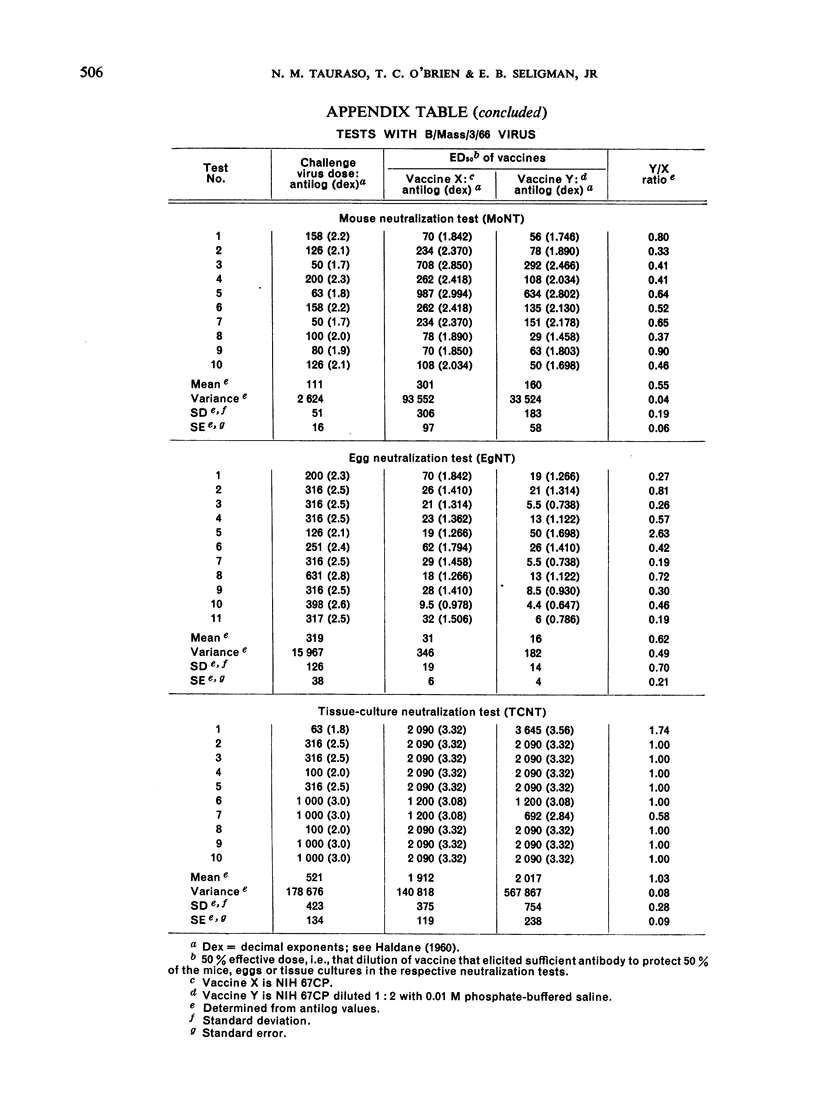
The data obtained in the second part of the mouse potency test, i.e., the neutralization test performed either in mice or in embryonated eggs, statistically demonstrated protective differences between 2 vaccines differing in antigenic mass by as little as 2-fold. However, the dependence upon a single egg or mouse neutralization test to provide the correct vaccine/reference ratio assumed more than ”biological” variation would allow. Further, the test was long and tedious and it would be impracticable to perform the number of tests needed to obtain statistically significant results. Thus, the extreme variability observed between individual mouse potency tests and the impracticability of performing this test in statistically sufficient numbers precluded its use for measuring antigenic content of inactivated influenza vaccines.
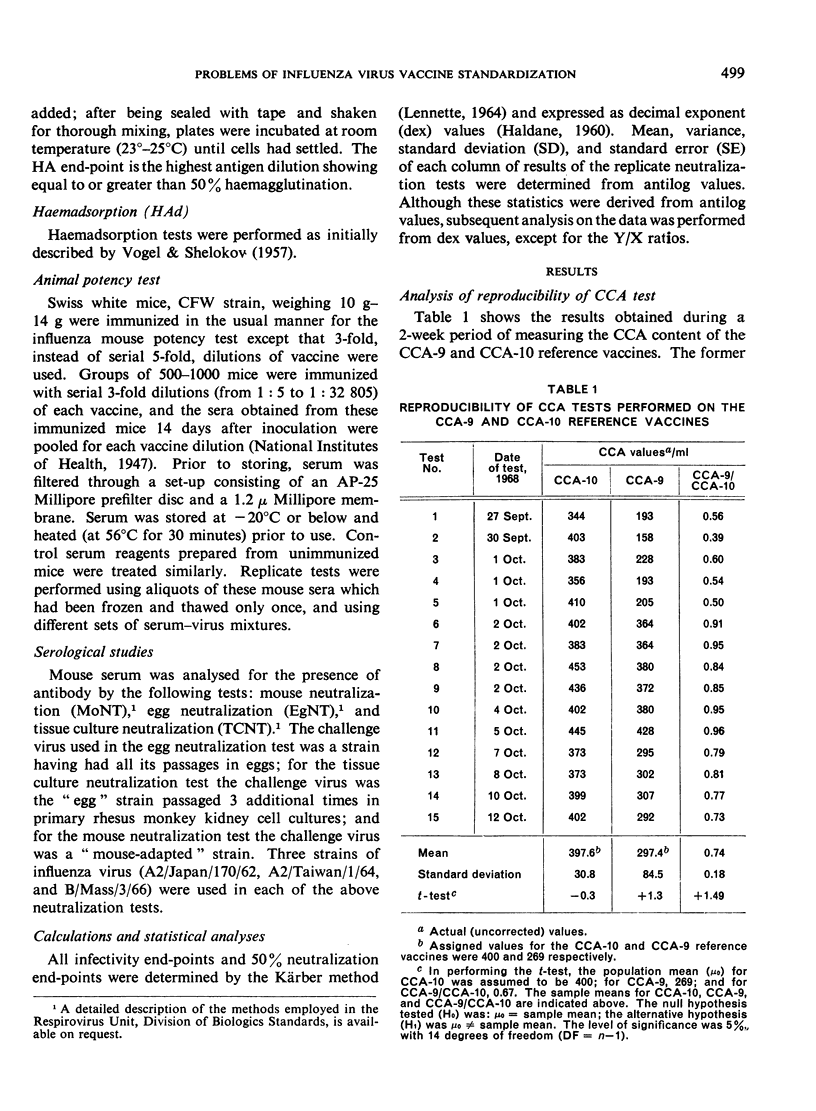
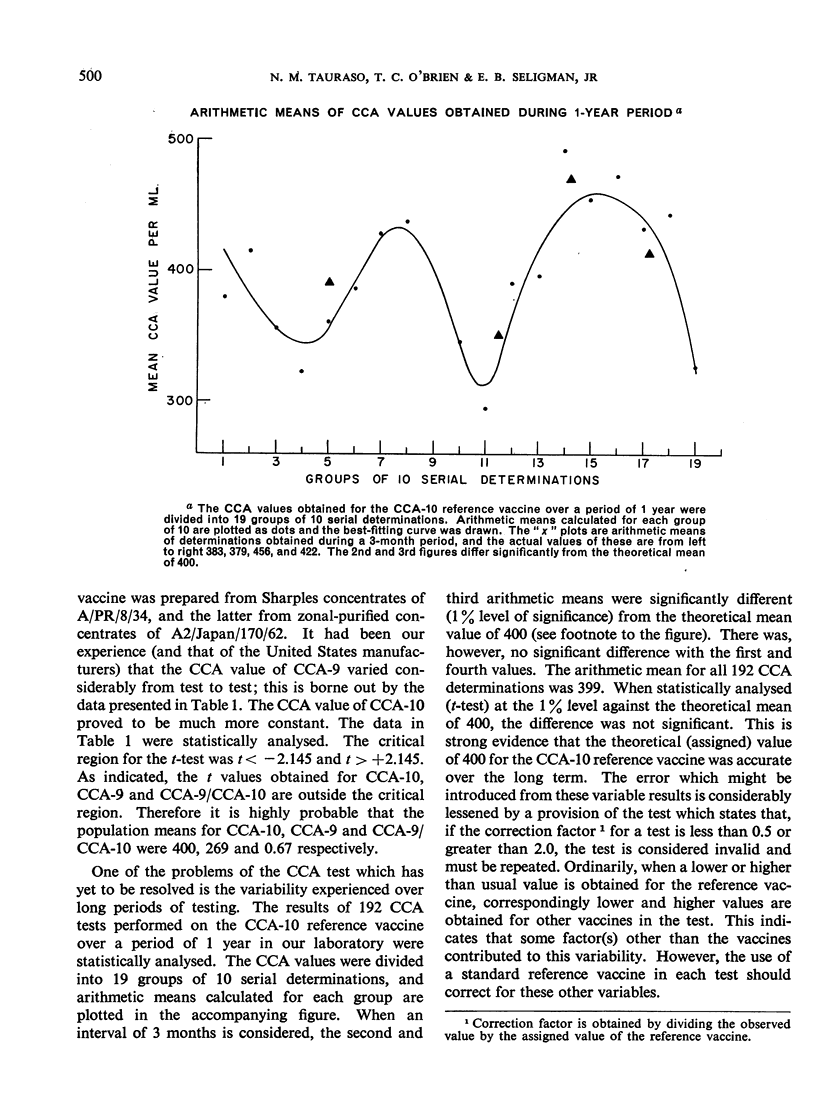
The simpler CCA test, on the other hand, did provide the reproducibility required for the correct determination of the vaccine/reference ratio once a stable CCA reference vaccine was prepared. This test was easily reproducible and results obtained were sufficient to allow a meaningful and reliable conclusion to be drawn with respect to vaccine potency.
The problems of measuring the relative content of several components in multivalent vaccine preparations and of finding a test which positively correlates with vaccine potency in man, however, remain unsolved.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hebeka E. K., Walker R. M., Beardmore W. B. Automated procedure for measuring antigenicity of extracted and intact influenza virus. Appl Microbiol. 1968 Nov;16(11):1699–1705. doi: 10.1128/am.16.11.1699-1705.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. K. STUDIES ON THE MECHANISM OF ADAPTATION OF INFLUENZA VIRUS TO MICE. J Exp Med. 1947 Oct 31;86(5):357–366. doi: 10.1084/jem.86.5.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. K. THE AGGLUTINATION OF RED CELLS BY ALLANTOIC FLUID OF CHICK EMBRYOS INFECTED WITH INFLUENZA VIRUS. Science. 1941 Jul 4;94(2427):22–23. doi: 10.1126/science.94.2427.22. [DOI] [PubMed] [Google Scholar]
- Hirst G. K. THE QUANTITATIVE DETERMINATION OF INFLUENZA VIRUS AND ANTIBODIES BY MEANS OF RED CELL AGGLUTINATION. J Exp Med. 1942 Jan 1;75(1):49–64. doi: 10.1084/jem.75.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE S., PUCK T. T., SAGIK B. P. An absolute method for assay of virus hemagglutinins. J Exp Med. 1953 Dec;98(6):521–531. doi: 10.1084/jem.98.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. L. Improved measurement of influenza virus hemagglutinin titer. J Immunol. 1965 Aug;95(2):336–344. [PubMed] [Google Scholar]
- Miller G. L., Stanley W. M. QUANTITATIVE ASPECTS OF THE RED BLOOD CELL AGGLUTINATION TEST FOR INFLUENZA VIRUS. J Exp Med. 1944 Feb 1;79(2):185–195. doi: 10.1084/jem.79.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- VOGEL J., SHELOKOV A. Adsorption-hemagglutination test for influenza virus in monkey kidney tissue culture. Science. 1957 Aug 23;126(3269):358–359. doi: 10.1126/science.126.3269.358-a. [DOI] [PubMed] [Google Scholar]
- WEISKRANTZ L. Effects of medial temporal lesions on taste preference in the monkey. Nature. 1960 Sep 3;187:879–880. doi: 10.1038/187879b0. [DOI] [PubMed] [Google Scholar]


