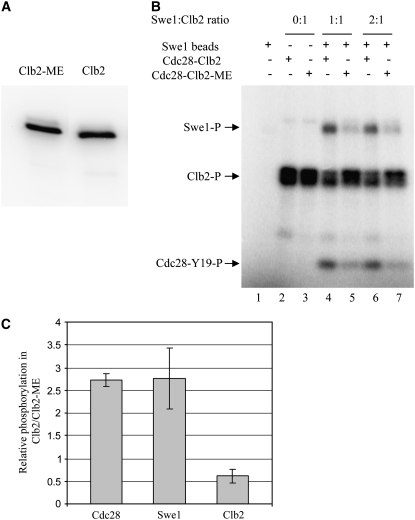
Figure 6.—
Clb2-ME disrupts mutual phosphorylations between Swe1 and Clb2-Cdc28 in vitro. Clb2-Ha3 and Clb2-ME-Ha3 were isolated from strains YGy65 (GAL-CLB2-HA3 swe1Δ) and YGy54 (GAL-CLB2-ME-HA3 swe1Δ), respectively, by immunoprecipitation and peptide elution (see materials and methods). (A) Eluates were analyzed by immunoblotting with anti-Clb2 antibody. (B) Swe1-Myc6 was isolated by immunoprecipitation from strain YGy29 (GAL-SWE1-MYC6) and incubated alone or with Clb2-Ha3 or Clb2-ME-Ha3, as indicated, all in the presence of [γ-32P]ATP. Samples were resolved by SDS–PAGE and phosphorylated Swe1, Clb2, and Cdc28 were detected by phosphorimaging. (C) Cdc28, Swe1, and Clb2 (or Clb2-ME) phosphorylation levels were quantified by standardization against the amount of Clb2 (or Clb2-ME) input (lanes 2 and 3) and plotted as the relative level of phosphorylation of each protein in the Clb2- vs. Clb2-ME-containing reactions. Error bars indicate standard deviation (n = 4).