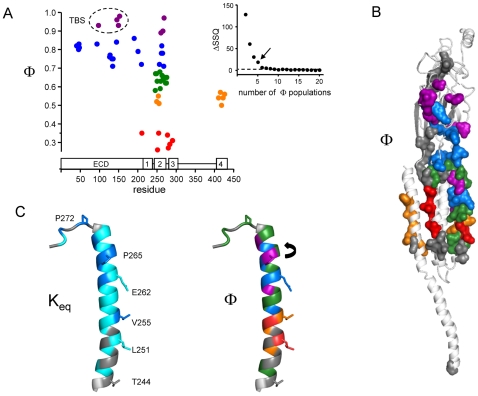
Figure 5. Keq and Φ of the α subunit.
(A) Population analysis of Φ in the α subunit. Φ-values of 55 different residues plotted as a function of sequence position (≥2 mutants and >5-fold range in Keq). Subunit domains are shown along the x-axis. Each residue was assigned to a Φ population by using a statistical algorithm (see below and Methods). The population means are: purple, 0.94; blue, 0.78; green, 0.64; orange, 0.54 and red, 0.31. Φ-values (Table 2) may reflect the relative timing of gating movements: purple/blue is early, green is intermediate and orange/red is late. High-Φ residues in the TBS are circled. Inset, The number of Φ populations (n) was estimated from the sum-squares deviation (SSQ). SSQ decreases significantly as n is increased from n = 2–5, but decreases more slowly between n = 6–20. The most likely number of Φ populations is 5. (B) Map of Φ in the α subunit. Residues are colored according to Φ value (see panel A for color code). The TBS and M2-cap (purple) move at the outset, and the equatorial residues (red) move near the end, of the channel-opening process. (C) Functional maps of αM2 and αM2-M3 linker (α244–α276). M2 residues T244, L251 and E262 face the lumen of the pore. Left, Residues colored according to the range for the fold-change in Keq: >1000-fold (blue), 10–1000 fold (cyan) and <10-fold (grey) (Table 2). αM2-cap residues experience large energy differences (‘move’) between C and O, whereas many mutants of residues near the cytoplasmic limit of the channel are iso-energetic, which may indicate relatively smaller structural changes. The three biggest excursions in Keq were observed for αP272, αP265 and αV255. Right, residues colored according to Φ value (see panel A for color code). Most of the residues in the αM2-cap move ‘early’ in gating (purple and blue), before those in the M2-M3 linker and much of M2 (green). Three cap residues (αI260, αP265 and αS268) have the same Φ value as those for residues at the transmitter binding sites (see panel A). In αM2, residues near the equator have the lowest Φ values and, therefore, move last in C→O gating. Arrow, we speculate that when the channel opens, αP265 rotates to position its side chain in the lumen of the channel.