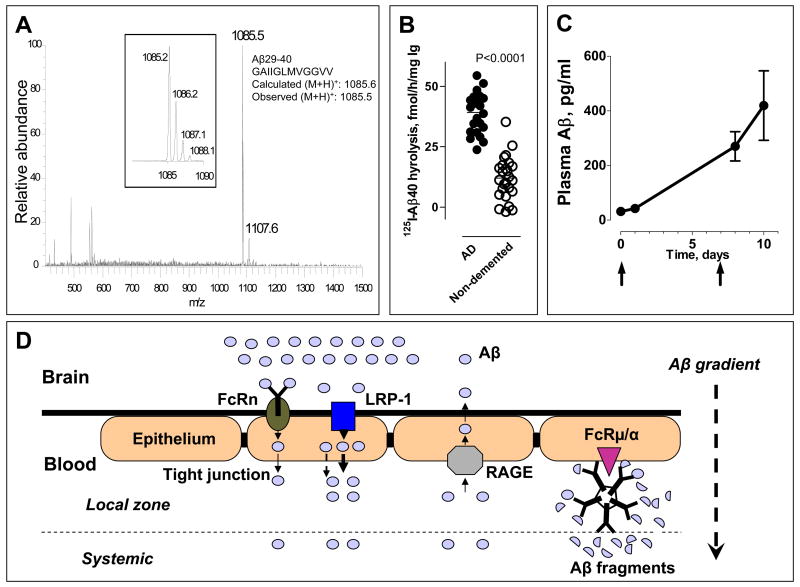
Fig 1.
(A) Aβ40 peptide bonds hydrolyzed by monoclonal IgM Yvo. ESI-MS spectrum of Aβ fragments generated by IgM. Inset, Zoom scan of spectrum region around m/z peak 1085.5, corresponding to the exact theoretical m/z for singly charged (M+H)+ ion of Aβ29-40. The peak-splitting in the zoom scan reflects the natural isotopic distribution of the singly charged peptide ions. (B) Increased Aβ hydrolysis by IgMs from patients with Alzheimer’s disease. Shown are values of 125I-Aβ1-40 (0.1 nM) hydrolysis (means of duplicates) incubated with the IgM preparations (0.023 mg/ml) purified from AD patients (n=23) and elderly, non-demented control subjects (n=25). Each point represents a different human subject. 2-tailed unpaired t-test.(C) Plasma Aβ mobilization induced by peripheral catalytic IgM administration. Pooled polyclonal IgM from human subjects purified by affinity chromatography using immobilized anti-IgM antibody was injected (360 μg) intravenously into APP/PS1-Tg mice (n=3) on day 0 and 7. Plasma Aβ was measured before and after IgM administration by an ELISA kit that detects intact Aβ40. The Aβ hydrolyzing activity of the human IgM is described in ref 27. (D) Hypothesis of catalytic IgM accelerated Aβ efflux from the brain. Hydrolysis of Aβ by peripherally IgM increases the Aβ brain-periphery concentration difference and thereby enhances Aβ efflux from the brain. Catalytic IgM bound to FcRμ/α on the luminal (blood) side of the blood-brain-barrier will accentuate the local concentration difference and may facilitate sustained brain Aβ depletion. FcRμ/α, Fc receptor for IgM. FcRn, neonatal Fc receptor. LRP-1, low-density lipoprotein receptor related protein 1; RAGE, receptor for advanced glycation end products.