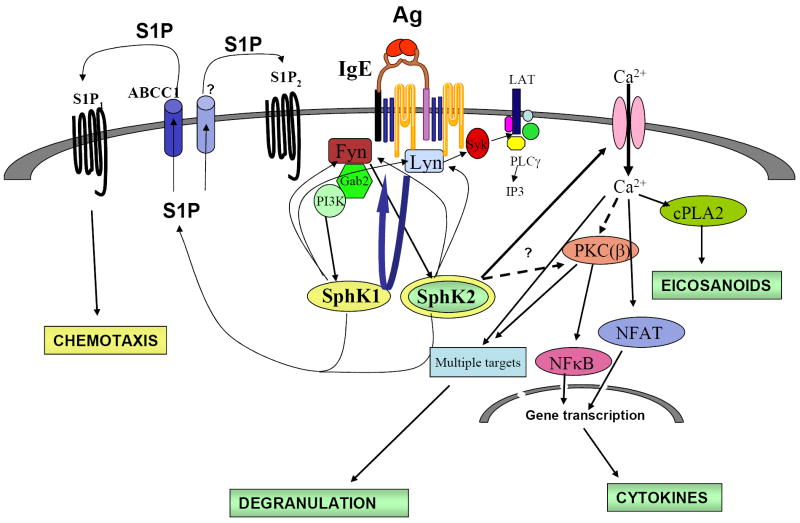
FIGURE 3. Activation and function of sphingosine kinases in murine bone-marrow derived mast cells.
After FcεRI engagement, the membrane-localized Src Kinases Lyn and Fyn form complexes with SphKs. This localizes SphKs to the membrane and to lipid rafts, where their substrate, SPH, is enriched. Fyn is necessary also for the activation of SphK1 and SphK2. Activated Fyn provides both Gab2/PI3K-dependent and -independent signals that are key to full activation of SphK1 and SphK2, respectively. The dependence on PI3K can be related to a direct effect of PIP3 on SphKs, its importance in PLD activation and phosphatidic acid generation, or other downstream signaling partners. SphK activities, in turn, may enhance Fyn and Lyn activities. SphK2 is essential for FcεRI-induced calcium influx from the extracellular media, PKC activation and consequent NFκB activation and thus, it affects degranulation, arachidonic acid, leukotriens and cytokine production. Furthermore, S1P production is required for the transactivation of the receptors S1P1 and S1P2. Presence of S1P2 is important for proper degranulation, and S1P1 for the to the movement of mast cells towards an Ag gradient. In murine mast cells, both SphK1 and SphK2 are necessary for chemotaxis towards Ag. An ABCC1-type of transporter is involved in the secretion of S1P into the media and in the transactivation of S1P1 but not S1P2. Other transporters may also participate in the secretion of S1P. Mast cell secreted S1P can promote inflammation by activating and recruiting other immune cells involved in allergic and inflammatory responses.