Scheme 2.
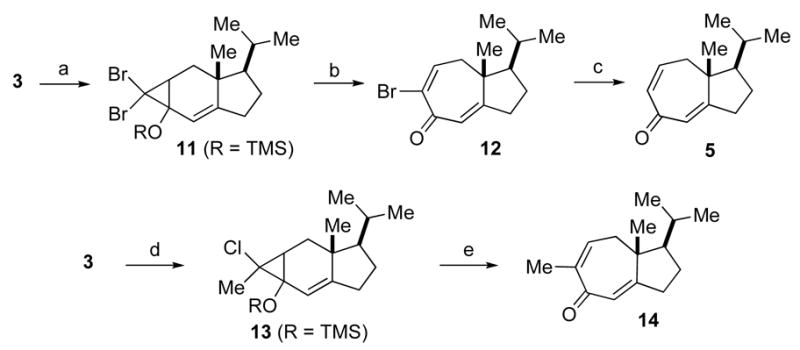
Synthesis of the ring expanded compounds 12 and 14. Reagents and conditions: (a) i) LDA, TMSC1, THF, −78 °C. ii) CHBr3, KOtBu, pentane 0 °C to 23 °C. (b) AgNO3, pyr, EtOH, 23 °C, 1.5 h, 59% (3 steps from 3). (c) BF3·OEt2, NaI, CH3CN, 0 °C, 41%. (d) i) LDA, TMSC1, THF, −78 °C. ii) dichloroethane, nBuLi, THF, −35 °C to 0 °C. (e) AgNO3, pyr, EtOH, 60 °C, 3.5 h, 45% (3 steps from 3).
